Interventions for preventing and treating cardiac complications in Duchenne and Becker muscular dystrophy and X-linked dilated cardiomyopathy
- PMID: 30326162
- PMCID: PMC6517009
- DOI: 10.1002/14651858.CD009068.pub3
Interventions for preventing and treating cardiac complications in Duchenne and Becker muscular dystrophy and X-linked dilated cardiomyopathy
Abstract
Background: The dystrophinopathies include Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and X-linked dilated cardiomyopathy (XLDCM). In recent years, co-ordinated multidisciplinary management for these diseases has improved the quality of care, with early corticosteroid use prolonging independent ambulation, and the routine use of non-invasive ventilation signficantly increasing survival. The next target to improve outcomes is optimising treatments to delay the onset or slow the progression of cardiac involvement and so prolong survival further.
Objectives: To assess the effects of interventions for preventing or treating cardiac involvement in DMD, BMD, and XLDCM, using measures of change in cardiac function over six months.
Search methods: On 16 October 2017 we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE and Embase, and on 12 December 2017, we searched two clinical trials registries. We also searched conference proceedings and bibliographies.
Selection criteria: We considered only randomised controlled trials (RCTs), quasi-RCTs and randomised cross-over trials for inclusion. In the Discussion, we reviewed open studies, longitudinal observational studies and individual case reports but only discussed studies that adequately described the diagnosis, intervention, pretreatment, and post-treatment states and in which follow-up lasted for at least six months.
Data collection and analysis: Two authors independently reviewed the titles and abstracts identified from the search and performed data extraction. All three authors assessed risk of bias independently, compared results, and decided which trials met the inclusion criteria. They assessed the certainty of evidence using GRADE criteria.
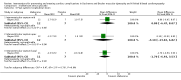
Main results: We included five studies (N = 205) in the review; four studies included participants with DMD only, and one study included participants with DMD or BMD. All studied different interventions, and meta-analysis was not possible. We found no studies for XLDCM. None of the trials reported cardiac function as improved or stable cardiac versus deteriorated.The randomised first part of a two-part study of perindopril (N = 28) versus placebo (N = 27) in boys with DMD with normal heart function at baseline showed no difference in the number of participants with a left ventricular ejection fraction (LVEF%) of less than 45% after three years of therapy (n = 1 in each group; risk ratio (RR) 1.04, 95% confidence interval (CI) 0.07 to 15.77). This result is uncertain because of study limitations, indirectness and imprecision. In a non-randomised follow-up study, after 10 years, more participants who had received placebo from the beginning had reduced LVEF% (less than 45%). Adverse event rates were similar between the placebo and treatment groups (low-certainty evidence).A study comparing treatment with lisinopril versus losartan in 23 boys newly diagnosed with Duchenne cardiomyopathy showed that after 12 months, both were equally effective in preserving or improving LVEF% (lisinopril 54.6% (standard deviation (SD) 5.19), losartan 55.2% (SD 7.19); mean difference (MD) -0.60% CI -6.67 to 5.47: N = 16). The certainty of evidence was very low because of very serious imprecision and study limitations (risk of bias). Two participants in the losartan group were withdrawn due to adverse events: one participant developed an allergic reaction, and a second exceeded the safety standard with a fall in ejection fraction greater than 10%. Authors reported no other adverse events related to the medication (N = 22; very low-certainty evidence).A study comparing idebenone versus placebo in 21 boys with DMD showed little or no difference in mean change in cardiac function between the two groups from baseline to 12 months; for fractional shortening the mean change was 1.4% (SD 4.1) in the idebenone group and 1.6% (SD 2.6) in the placebo group (MD -0.20%, 95% CI -3.07 to 2.67, N = 21), and for ejection fraction the mean change was -1.9% (SD 9.8) in the idebenone group and 0.4% (SD 5.5) in the placebo group (MD -2.30%, 95% CI -9.18 to 4.58, N = 21). The certainty of evidence was very low because of study limitations and very serious imprecision. Reported adverse events were similar between the treatment and placebo groups (low-certainty evidence).A multicentre controlled study added eplerenone or placebo to 42 patients with DMD with early cardiomyopathy but preserved left ventricular function already established on ACEI or ARB therapy. Results showed that eplerenone slowed the rate of decline of magnetic resonance (MR)-assessed left ventricular circumferential strain at 12 months (eplerenone group median 1.0%, interquartile range (IQR) 0.3 to -2.2; placebo group median 2.2%, IQR 1.3 to -3.1%; P = 0.020). The median decline in LVEF over the same period was also less in the eplerenone group (-1.8%, IQR -2.9 to 6.0) than in the placebo group (-3.7%, IQR -10.8 to 1.0; P = 0.032). We downgraded the certainty of evidence to very low for study limitations and serious imprecision. Serious adverse events were reported in two patients given placebo but none in the treatment group (very low-certainty evidence).A randomised placebo-controlled study of subcutaneous growth hormone in 16 participants with DMD or BMD showed an increase in left ventricular mass after three months' treatment but no significant improvement in cardiac function. The evidence was of very low certainty due to imprecision, indirectness, and study limitations. There were no clinically significant adverse events (very low-certainty evidence).Some studies were at risk of bias, and all were small. Therefore, although there is some evidence from non-randomised data to support the prophylactic use of perindopril for cardioprotection ahead of detectable cardiomyopathy, and for lisinopril or losartan plus eplerenone once cardiomyopathy is detectable, this must be considered of very low certainty. Findings from non-randomised studies, some of which have been long term, have led to the use of these drugs in daily clinical practice.
Authors' conclusions: Based on the available evidence from RCTs, early treatment with ACE inhibitors or ARBs may be comparably beneficial for people with a dystrophinopathy; however, the certainty of evidence is very low. Very low-certainty evidence indicates that adding eplerenone might give additional benefit when early cardiomyopathy is detected. No clinically meaningful effect was seen for growth hormone or idebenone, although the certainty of the evidence is also very low.
Conflict of interest statement
John Bourke is a Consultant cardiologist and principal investigator for a multicentre, placebo‐controlled trial for cardiac protection in DMD
Teofila Bueser is a specialist nurse and manages patients with DMD, BMD and X‐linked muscular dystrophy. She has no conflicts of interest.
Dr Quinlivan has received honoraria from PTC bio for teaching on ataluren and Santhera for teaching on idebenone. She is Joint Co‐ordinating Editor of Cochrane Neuromuscular. She was not involved in the editorial process for this review.
Figures












Comment in
-
What are the effects of treatments used to prevent or treat heart complications in Duchenne muscular dystrophy, Becker muscular dystrophy, and X-linked dilated cardiomyopathy? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2020 Jul;62(7):776-777. doi: 10.1111/dmcn.14568. Epub 2020 May 19. Dev Med Child Neurol. 2020. PMID: 32427344 No abstract available.
References
References to studies included in this review
Allen 2013 {published data only}
-
- Allen HD, Flanigan KM, Thrush PT, Viollet‐Callendret L, Dvorchik I, Yin H, et al. A randomized, double‐blind trial of lisinopril and losartan for the treatment of cardiomyopathy in Duchenne muscular dystrophy. PLOS Currents Muscular Dystrophy 2013;5:1‐13. [PMCID: PMC3871420; PUBMED: 24459612] - PMC - PubMed
-
- NCT01982695. Cardiomyopathy in DMD: lisinopril vs. losartan [Compare efficacy of the angiotensin converting enzyme inhibitor (ACEI) lisinopril with angiotensin II receptor antagonist losartan (ARB) for the cardiomyopathy of Duchenne muscular dystrophy]. clinicaltrials.gov/ct2/show/study/NCT01982695 First received: 29 October 2013; results first received 14 August 2015.
Buyse 2011 {published data only}
-
- Buyse GM, Goemans N, Hauwe M, Thijs D, Groot IJM, Schara U, et al. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: results from a randomized placebo‐controlled trial. Neuromuscular Disorders 2011;21:396‐405. [PUBMED: 21435876] - PubMed
Cittadini 2003 {published data only}
-
- Cittadini A, Ines Comi L, Longobardi S, Rocco Petretta V, Casaburi C, Passamano L, et al. A preliminary randomized study of growth hormone administration in Becker and Duchenne muscular dystrophies. European Heart Journal 2003;24(7):664‐72. [PUBMED: 12657225] - PubMed
Duboc 2005 {published data only}
-
- Duboc D, Meune C, Lerebours G, Devaux J‐Y, Vaksmann G, Bécane H‐M. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. Journal of the American College of Cardiology 2005;45(6):855‐7. [PUBMED: 15766818] - PubMed
References to studies excluded from this review
Bushby 2014 {published data only}
Duboc 2007 {published data only}
-
- Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain T, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years follow‐up. American Heart Journal 2007;154(3):596‐602. [PUBMED: 17719312] - PubMed
Folkers 1985 {published data only}
Ishikawa 1995 {published data only}
-
- Ishikawa Y, Back J, Ishikawa Y, Minami R. A management trial for Duchenne cardiomyopathy. American Journal of Physical Medicine & Rehabilitation 1995;74:345‐50. - PubMed
Kajimoto 2006 {published data only}
-
- Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, et al. Beta‐blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circulation Journal 2006;70(8):991‐4. - PubMed
Matsumura 2010 {published data only}
-
- Matsumura T, Tamura T, Kuru S, Kikuchi Y, Mitsuru K. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Internal Medicine 2010;49(14):1357‐63. - PubMed
Mendell 2013 {published data only}
-
- Mendell J, Rodino‐Klapac L, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen Study Group. Eteplirsen for the treatment of DMD. Annals of Neurology 2013;74(5):637‐47. - PubMed
Rhodes 2008 {published data only}
-
- Rhodes J, Margossian R, Darras BT, Colan SD, Jenkins KJ, Geva T, Powell AJ. Safety and efficacy of carvedilol therapy for patients with dilated cardiomyopathy secondary to muscular dystrophy. Pediatric Cardiology 2008;29(2):343‐51. - PubMed
Voit 2014 {published data only}
-
- Voit T, Topaloglu H, Straub V, Muntoni F, Deconinck N, Campion G, et al. Safety and efficacy of Drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised placebo controlled phase II study. Lancet Neurology 2014;13(10):987‐96. - PubMed
References to studies awaiting assessment
EUCTR2008‐007236‐18‐IT {published data only}
-
- EUCTR2008‐007236‐18‐IT. Effects of cardioprotective therapy, carvedilol vs ramipril, in patients affected by Duchenne and Becker muscular dystrophy. Clinical significance and prognostic value of cardiac magnetic resonance study. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2008‐00... (first received 11 December 2008).
Leung 2014 {published data only}
-
- NCT01168908. Revatio for heart disease in Duchenne muscular dystrophy and Becker muscular dystrophy (REVERSE‐DBMD). clinicaltrials.gov/show/NCT01168908 (first received 23 July 2010).
Salehi 2017 {published data only}
-
- IRCT2015070223018N1. Effect of Q10 coenzyme in improving cardiac function [A study on the effect of Q10 coenzyme via tissue doppler method in improving cardiac function in patients with early Duchenne cardiomyopathy aged 6‐10 years]. en.irct.ir/trial/19746 (first received 11 August 2015).
References to ongoing studies
FOR‐DMD 2012 {published data only}
-
- NCT01603407. Finding the optimum regimen for Duchenne muscular dystrophy (FOR‐DMD) [Duchenne muscular dystrophy: double‐blind randomized trial to find optimum steroid regimen]. https://clinicaltrials.gov/ct2/show/NCT01603407 (first received 23 May 2012).
ISRCTN50395346 {published data only}
-
- ISRCTN50395346. A double‐blind randomised multi‐centre, placebo‐controlled trial of combined angiotensin converting enzyme‐inhibitor and beta‐blocker therapy in preventing the development of cardiomyopathy in genetically characterised males with Duchenne muscular dystrophy without echo‐detectable left ventricular dysfunction. isrctn.com/ISRCTN50395346 (first received 13 August 2007).
NCT00606775 {published data only}
-
- NCT00606775. The preventive efficacy of carvedilol on cardiac dysfunction in Duchenne muscular dystrophy [Carvedilol for the prevention of minor cardiac damage and cardiac function in Duchenne muscular dystrophy]. clinicaltrials.gov/show/NCT00606775 (first received 5 February 2008).
NCT00819845 {published data only}
-
- NCT00819845. Ramipril versus carvedilol in Duchenne and Becker patients [Effects of cardioprotective therapy, carvedilol vs ramipril, in patients affected by Duchenne and Becker muscular dystrophy. Clinical significance and prognostic value of cardiac magnetic resonance study]. clinicaltrials.gov/show/NCT00819845 (first received 9 January 2009).
NCT01126697 {published data only}
-
- NCT01126697. Clinical trial of coenzyme Q10 and lisinopril in muscular dystrophies [PITT0908:clinical trial of coenzyme Q10 and lisinopril in muscular dystrophies]. clinicaltrials.gov/show/NCT01126697 (first received 20 May 2010).
NCT01350154 {published data only}
-
- NCT01350154. Effect of modulating the nNOS system on cardiac, muscular and cognitive function in Becker muscular dystrophy patients [Does modulation of the nNOS system in patients with muscular dystrophy and defect nNOS signalling affect cardiac, muscular or cognitive function?]. clinicaltrials.gov/show/NCT01350154 (first received 9 May 2011).
NCT01648634 {published data only}
-
- NCT01648634. Nebivolol for the prevention of left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy (NEBIDYS) [A randomized, double‐blind, placebo‐controlled, multi‐center study to examine the effect of nebivolol, a beta‐blockade drug, for the prevention of ventricular systolic dysfunction in patients with Duchenne muscular dystrophy]. clinicaltrials.gov/show/NCT01648634 (first received 24 July 2012).
NCT02354352 {published data only}
-
- NCT02354352. Therapeutic potential for aldosterone inhibition in Duchenne muscular dystrophy. https://clinicaltrials.gov/ct2/show/NCT02354352 (first received 21 August 2018).
NCT02432885 {published data only}
-
- NCT02432885. Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy ‐ ACE inhibitor therapy trial [Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy ‐ angiotensin‐converting‐enzyme (ACE) inhibitor therapy]. clinicaltrials.gov/show/NCT02432885 (first received 4 May 2015).
NCT02485938 {published data only}
-
- NCT02485938. HOPE‐Duchenne (Halt cardiomyOPathy progrEssion in Duchenne) (HOPE) [A randomized, open‐label study of the safety and efficacy of multi‐vessel intracoronary delivery of allogeneic cardiosphere‐derived cells in patients with cardiomyopathy secondary to Duchenne muscular dystrophy]. clinicaltrials.gov/show/NCT02485938 (first received 30 June 2015).
NCT03340675 {published data only}
-
- NCT03340675. Oral ifetroban in subjects with Duchenne muscular dystrophy (DMD) [A randomized, double‐blind, placebo‐controlled, multiple dose study to determine the safety, pharmacokinetics and efficacy of oral ifetroban in subjects with Duchenne muscular dystrophy (DMD)]. clinicaltrials.gov/show/NCT03340675 (first received 13 November 2017).
NCT03406780 {published data only}
-
- NCT03406780. A study of CAP‐1002 in ambulatory and non‐ambulatory patients with Duchenne muscular dystrophy (HOPE‐2) [A phase 2, randomized, double‐blind, placebo‐controlled trial evaluating the safety and efficacy of intravenous delivery of allogeneic cardiosphere‐derived cells in subjects with Duchenne muscular dystrophy]. https://clinicaltrials.gov/ct2/show/NCT03406780 (first received 23 January 2018).
NCT03439670 {published data only}
-
- NCT03439670. A study to assess the efficacy and safety of vamorolone in boys with Duchenne muscular dystrophy (DMD) [A phase IIb randomized, double‐blind, parallel group, placebo‐ and active‐controlled study with double‐blind extension to assess the efficacy and safety of vamorolone in ambulant boys with Duchenne muscular dystrophy (DMD)]. https://clinicaltrials.gov/ct2/show/NCT03439670 (first received 24 July 2018).
Additional references
Aartsma‐Rus 2013
-
- Aartsma‐Rus A, Ommen GJ, Kaplan JC. Innovating therapies for muscle diseases. Handbook of Clinical Neurology 2013;113:1497‐501. - PubMed
Abdel‐Salam 2014
-
- Abdel‐Salam Z, Rayan M, Saleh A, Abdel‐Barr MG, Hussain M, Nammas W. I(f) current inhibitor ivabradine in patients with idiopathic dilated cardiomyopathy: impact on the exercise tolerance and quality of life. Cardiology Journal 2014;22(2):227‐32. - PubMed
Abraham 2014
-
- Abraham WT, Aggarwal S, Prabhu SD, Cecere R, Pamboukian SV, Bank AJ, et al. C‐Pulse Trial Group. Ambulatory extra‐aortic counterpulsation in patients with moderate to severe chronic heart failure. JACC: Heart Failure 2014;2(5):526‐33. - PubMed
Adorisio 2017
-
- Adorisio R, Catarutti N, D'Amico A, Chinali M, Baban A, Iorio FS, et al. Heart rate reduction strategy with Ivabradine in reducing acute heart failure in Duchenne dilated cardiomyopathy. European Heart Journal 2017;38(Suppl 1):5275.
Andrikopoulos 2013
-
- Andrikopoulos G, Kourouklis S, Trika C, Tzeis S, Rassias I, Papademetriou C, et al. Cardiac resynchronisation therapy in Becker muscular dystrophy. Hellenic Journal of Cardiology 2013;54(3):227‐9. - PubMed
Angelini 1996
-
- Angelini C, Fanin M, Freda MP, Martinello F, Miorin M, Melacini P, et al. Prognostic factors in mild dystrophinopathies. Journal of the Neurological Sciences 1996;142(1‐2):70‐8. - PubMed
Backman 1992
-
- Backman E, Nylander E. The heart in Duchenne muscular dystrophy: a non‐invasive study. European Heart Journal 1992;13(9):1239‐44. - PubMed
Barber 2013
-
- Barber B, Andrews J, Lu Z, West N, Meaney J, Price E, et al. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. Journal of Pediatrics 2013;163(4):1080‐4. - PubMed
Becker 1955
-
- Becker PE, Kiener F. Eine neue X‐chromasomale Muskeldystrophie. Archiv fur Psychiatrie und Nervenkrankheiten 1955;193:427‐48. - PubMed
Bies 1992
-
- Bies RD, Friedman D, Roberts R, Perryman MB, Caskey CT. Expression and localization of dystrophin in human cardiac Purkinje fibres. Circulation 1992;86(1):147‐53. - PubMed
Biggar 2006
-
- Bigggar WD, Harris VA, Eliasoph L, Alman B. Long term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscular Disorders 2006;16(4):249‐55. - PubMed
Black 2016
-
- Black MC, Schumer EM, Rogers M, Trivedi J, Slaughter MS. Sunshine Heart C‐Pulse: device for NYHA III and ambulatory class IV heart failure. Future Cardiolology 2016;12(5):521‐31. - PubMed
Bleeker 2006
-
- Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006;113(7):969–76. - PubMed
Burnett 2017
Bushby 1991
-
- Bushby KMD, Thambyayah M, Gardner‐Medwin D. Prevalence and incidence of Becker muscular dystrophy. Lancet 1991;337(8748):1022‐4. - PubMed
Bushby 1993
-
- Bushby K, Goodship JA, Nicholson L, Johnson M, Haggerty ID, Gardner‐Medwin D. Variability in clinical, genetic and protein abnormalities in manifesting carriers of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders 1993;3(1):57‐64. - PubMed
Casazza 1988
-
- Casazza F, Brambilla G, Salvato A, Morandi L, Gronda E, Bonacina E. Cardiac transplantation in Becker muscular dystrophy. Journal of Neurology 1988;235(8):496‐8. - PubMed
Cevik 2010
Cicoira 2002
-
- Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zeni P, et al. Relation of aldosterone 'escape' despite angiotensin‐converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy. American Journal of Cardiology 2002;89(4):403‐7. - PubMed
Corrado 2002
-
- Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia‐Manzillo G, et al. Prognostic value of echocardiograms, ventricular late potentials, ventricular arrhythmias and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. American Journal of Cardiology 2002;89(7):838‐41. - PubMed
Danialou 2001
-
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Rosiers C, et al. Dystrophin‐deficient cardiomyocytes are abnormally vulnerable to mechanical stress‐induced contractile failure and injury. FASEB Journal 2001;15(9):1655‐7. - PubMed
De Kermadec 1994
-
- Kermadec JM, Bécane HM, Chénard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an electrocardiographic study. American Heart Journal 1994;127(3):618‐23. - PubMed
De Visser 1992
-
- Visser M, Voogt WG, Rivière GV. The heart in Becker muscular dystrophy, fascioscapulohumeral muscular dystrophy and Bethlem myopathy. Muscle & Nerve 1992;15(5):591‐6. - PubMed
Donofrio 1989
-
- Donofrio PD, Challa VR, Hackshaw BT, Mills SA, Cordell R. Cardiac transplant in a patient with muscular dystrophy and cardiomyopathy. Archives of Neurology 1989;46(6):705‐7. - PubMed
Eagle 2002
-
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disorders 2002;12(10):926‐9. - PubMed
Emery 1976
-
- Emery AEH, Skinner R. Clinical studies in benign (Becker type) X‐linked muscular dystrophy. Clinical Genetics 1976;10(4):189‐201. - PubMed
Emery 2003
-
- Emery AEH, Muntoni F. Duchenne Muscular Dystrophy. 3rd Edition. Oxford: Oxford Medical Publications, 2003.
Fararolo 2010
-
- Fararolo R, Peradejord M, Berlotti A, Diez M, Favaloro L, Gomez C, et al. Results of heart transplantation: 16 years experience in a center in Argentina. Transplantation Proceedings 2010;42(1):321‐3. - PubMed
Fayssoil 2008
-
- Fayssoil A, Orlikowski D, Nardi O, Annane D. Complete atrioventricular block in Duchenne muscular dystrophy. Europace 2008;10(11):1351‐2. - PubMed
Ferlini 1999
-
- Ferlini A, Sewry C, Melis MA, Mattedu A, Muntoni F. X‐linked dilated cardiomyopathy and the dystrophin gene. Neuromuscular Disorders 1999;9(5):339‐46. - PubMed
Finsterer 2003
-
- Finserer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology 2003;99(1):1‐19. - PubMed
Gardner 1995
Globus 1923
-
- Globus JH. The pathological findings in the heart muscle in progressive muscular dystrophy. Archives of Neurology and Psychiatry 1923;9(1):59‐72.
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed 20 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Griffin 2001
-
- Griffin JL, Williams HJ, Sang E, Clarke K, Rae C, Nicholson JK. Metabolic profiling of genetic disorders: a multi‐tissue (1)H nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue. Analytical Biochemistry 2001;293(1):16‐21. - PubMed
Heran 2012
Heymsfield 1978
-
- Heymsfield SB, McNish T, Perkins JV, Felner JM. Sequence of cardiac changes in Duchenne muscular dystrophy. American Heart Journal 1978;95(3):283‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holloway 2008
-
- Holloway SM, Wilcox DE, Wilcox A, Dean JC, Berg JN, Goudie DR, et al. Life expectancy and death from cardiomyopathy amongst carriers of Duchenne and Becker muscular dystrophy in Scotland. Heart 2008;94(5):633‐6. - PubMed
Hoogerwaard 1997
-
- Hoogerwaard EM, Voogt WG, Wilde AA, Wouw PA, Bakker E, Ommen GJ, et al. Evolution of cardiac abnormalities in Becker muscular dystrophy over a 13‐year period. Journal of Neurology 1997;244(10):657‐63. - PubMed
Hoogerwaard 1999
-
- Hoogerwaard EM, Wouw PA, Wilde AA, Bakker E, Ippel PF, Oosterwijk JC, et al. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders 1999;9(5):347‐51. - PubMed
Hor 2011
Hussain 2014
-
- Hussein G, Mansour L, Ghafor H, Mostafa F, Fawaz L. Short‐term effects of corticosteroid therapy on cardiac and skeletal muscles in muscular dystrophies. Journal of Investigative Medicine 2014;62(6):875‐9. - PubMed
Iodice 2015
-
- Iodice F, Testa G, Averardi M, Brancaccio G, Amodeo A, Cogo P. Implantation of a left ventricular assist device as a destination therapy in Duchenne muscular dystrophy patients with end stage cardiac failure: management and lessons learned. Neuromuscular Disorders 2015;25(1):19‐23. - PubMed
Ishigaki 1997
-
- Ishigaki C, Patria SI, Nishio H, Yoshioka A, Matsuo M. Early cardiac failure in a child with Becker muscular dystrophy is due to an abnormally low amount of dystrophin transcript lacking exon 13. Acta Paediatrica Japonica 1997;39(6):685‐9. - PubMed
Kamamura 1990
-
- Kamakura K, Kawai M, Arahata K, Koizuma H, Watanabe K, Sugita HA. A manifesting carrier of Duchenne muscular dystrophy with severe myocardial symptoms. Journal of Neurology 1990;237(8):483‐5. - PubMed
Koenig 1989
Kono 2015
Kuru 2012
-
- Kuru S, Tanahashi T, Matsumoto S, Kitamura T, Konagava M. Complete atrioventricular block in Duchenne muscular dystrophy. Rinsho Shinkeigaku 2012;52(9):684‐7. - PubMed
Kwon 2012
Lane 1980
-
- Lane RJ, Gardner‐Medwin D, Roses AD. Electrocardiographic abnormalities in carriers of Duchenne muscular dystrophy. Neurology 1980;30(5):497‐506. - PubMed
Lanza 2001
-
- Lanza GA, Dello Russo A, Giglio V, Luca L, Messano L, Santini C, et al. Impairment of cardiac autonomic function in patients with Duchenne muscular dystrophy: relationship to myocardial and respiratory function. American Heart Journal 2001;141(5):808‐12. - PubMed
Malhotra 1988
-
- Malhotra SB, Hart KA, Klamut HJ, Thomas NS, Bodrug SE, Burghes AH, et al. Frame‐shift deletions in patients with Duchenne and Becker muscular dystrophy. Science 1988;242(4879):755‐9. - PubMed
Matsumara 2010
-
- Matsumara T, Tamura T, Kuru S, Kikuchi Y, Kewai M. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Internal Medicine 2010;49:1357‐63. - PubMed
Matthews 2016
Melacini 1996
-
- Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, Miorin M, et al. Myocardial involvement is very frequent in patients affected with subclinical Becker's muscular dystrophy. Circulation 1996;94(12):3168‐75. - PubMed
Mendell 2012
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al‐Dahhak R, Gastier‐Foster J, et al. Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology 2012;71(3):304‐13. - PubMed
Menke 1991
-
- Menke A, Jockusch H. Decreased osmotic stability of dystrophin‐less muscle cells from the mdx mouse. Nature 1991;349(6304):69‐71. - PubMed
Meune 2004
Miyoshi 1991
Mori 2002
-
- Mori K, Manabe T, Nii M, Hayabuchi Y, Kuroda Y, Tatara K. plasma levels of Natriuretic peptides and echocardiographic parameters in Duchenne's muscular dystrophy. Paediatric Cardiology 2002;23(2):160‐6. - PubMed
Mori 2007
-
- Mori K, Hayabuchi Y, Inoue M, Suzuki M, Sakata M, Nakagawa R, et al. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne's progressive muscular dystrophy. Echocardiography 2007;24(6):598‐608. - PubMed
Mukoyama 1987
-
- Mukoyama M, Kondo K, Hizawa K, Nishitani H. Life spans of Duchenne muscular dystrophy patients in the hospital care programme in Japan. Journal of the Neurological Sciences 1987;81(2‐3):155‐8. - PubMed
Muntoni 1997
Muntoni 2003
-
- Muntoni F. Cardiomyopathy in muscular dystrophies. Current Opinion in Neurology 2003;16(5):577‐83. - PubMed
Nigro 1983
-
- Nigro G, Comi LI, Limongelli FM, Giugliano MA, Politano L, Petretta V. Prospective study of X‐linked progressive muscular dystrophy in Campania. Muscle & Nerve 1983;6(4):253‐62. - PubMed
Nigro 1990
-
- Nigro G, Comi L, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. International Journal of Cardiology 1990;26(3):271‐7. - PubMed
Nigro 1995
-
- Nigro G, Comi LI, Politano L, Limongelli FM, Nigro V, Rimini ML, et al. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle & Nerve 1995;18(3):283‐91. - PubMed
Nigro 2002
Nikolic 1998
-
- Nikolíc G. Dominant R wave in lead V1. Heart & Lung 1998;27(5):352‐3. - PubMed
Nolan 2003
-
- Nolan MA, Jones OD, Pedersen RL, Johnston HM. Cardiac assessment in childhood carriers of Duchenne and Becker muscular dystrophies. Neuromuscular Disorders 2003;13(2):129‐32. - PubMed
Olfors 1994
Papa 2017
Perloff 1984
-
- Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation 1984;69(1):33‐42. - PubMed
Phillips 2008
Ponikowski 2016
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2016;18(8):891‐975. - PubMed
Quinlivan 1995
-
- Quinlivan R, Ball J, Dunckley M Thomas DJ, Flinter F, Morgan‐Hughes J. Becker muscular dystrophy presenting with complete heart block in the sixth decade. Journal of Neurology 1995;242(6):398‐400. - PubMed
Quinlivan 1996
-
- Quinlivan RM, Lewis P, Marsden P, Dundas R, Robb SA, Baker E. Cardiac function, metabolism and perfusion in Duchenne and Becker muscular dystrophy. Neuromuscular Disorders 1996;6(4):237‐46. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2014
-
- Ryan TD, Jefferies JL, Sawnani H, Wong BL, Gardner A, Corral M, et al. Implantation of the HeartMate II and HeartWare left ventricular assist devices in patients with Duchenne muscular dystrophy: lessons learned from the first applications. ASAIO Journal 2014;60(2):246‐8. - PubMed
Sakata 1990
-
- Sakata C, Sunohara N, Nonaka I, Arahata K, Sugita H. A case of Becker muscular dystrophy presenting cardiac failure as the initial symptom. Rinsho Shinkeigaku 1990;30(2):210‐3. - PubMed
Schram 2013
-
- Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All‐cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. Journal of the American College of Cardiology 2013;61(9):948‐54. - PubMed
Silva 2007
-
- Silva MC, Meira ZM, Gurgel Giannetti J, Silva MM, Campos AF, Barbosa Mde M, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. Journal of the American College of Cardiology 2007;49(18):1874‐9. - PubMed
Silversides 2003
-
- Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. American Journal of Cardiology 2003;91(6):769‐72. - PubMed
Soslow 2016
Spurney 2015
Stabile 2013
Steare 1992
Swedberg 2010
Takenaka 1993
-
- Takenaka A, Yokota M, Iwase M, Miyaguchi K, Hayashi H, Saito H. Discrepancy between systolic and diastolic dysfunction of the left ventricle in patients with Duchenne muscular dystrophy. European Heart Journal 1993;14(5):669‐76. - PubMed
Tanaka 1979
-
- Tanaka H, Nishi S, Katanasako H. Natural course of cardiomyopathy in Duchenne muscular dystrophy. Japanese Circulation Journal 1979;43(11):974‐84. - PubMed
Towbin 1993
-
- Towbin JA, Hejtmancik JF, Brink P, Gelb B, Zhu XM, Chamberlain JS, et al. X‐linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation 1993;87(6):1854‐65. - PubMed
Turley 2008
-
- Turley A, Raja SG, Salhiyyah K, Nagarajan K. Does cardiac resynchronisation therapy improve survival and quality of life in patients with end‐stage heart failure?. Interactive Cardiovascular and Thoracic Surgery 2008;7(6):141‐6. - PubMed
Varni 1999
-
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical Care 1999;37(2):126‐39. [PUBMED: 10024117] - PubMed
Vincent 2007
-
- Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for Neuromuscular Disease (INQol). Neurology 2007;68(13):1051‐7. - PubMed
Viollet 2012
-
- Viollet L, Thrush PT, Flanigan KM, Mendell JR, Allen HD. Effects of angiotensin‐converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. American Journal of Cardiology 2012;110(1):98‐102. - PubMed
Wagner 2007
-
- Wagner K, Lechtzin N, Judge D. Current treatment of adult Duchenne muscular dystrophy. Biochemica et Biophysica Acta 2007;1772:229‐237. - PubMed
Ware 2007
-
- Ware JE Jr, Kosinski M, Bjorner JB, Turner‐Bowker DM, Gandek B, Maruish ME. User's Manual for the SF‐36v2TM Health Survey. 2nd Edition. Lincoln, RI: Quality Metric Incorporated, 2007.
Wasala 2013
Wu 2010
-
- Wu R, Gupta S, Brown R, Yancy C, Wald J, Kaiser P, et al. Clinical outcomes after cardiac transplantation in muscular dystrophy patients. Journal of Heart and Lung Transplantation 2010;29(4):432‐8. - PubMed
Yamamoto 1988
-
- Yamamoto S, Matsushima H, Suzuki A, Sotobata I, Indo T, Matsuoka Y. A comparative study of thallium‐201 single‐photon emission computed tomography and electrocardiography in Duchenne and other types of muscular dystrophy. American Journal of Cardiology 1988;61(10):838‐43. - PubMed
Zannad 2000
-
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000;102(6):2700‐6. - PubMed
References to other published versions of this review
Quinlivan 2011
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

