Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent
- PMID: 30326276
- PMCID: PMC10321369
- DOI: 10.1016/j.actbio.2018.10.018
Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent
Abstract
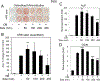
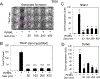
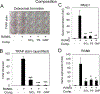
Silica based nanoparticles have been demonstrated to have intrinsic biologic activity towards the skeleton and to function by promoting the differentiation of bone forming osteoblasts while inhibiting the differentiation of bone resorbing osteoclasts. The excitement surrounding nanomedicine in part revolves around the almost unlimited possibilities for varying the physicochemical properties including size, composition, and surface charge. To date few studies have attempted to manipulate these characteristics in concert to optimize a complex biologic outcome. Towards this end, spherical silica nanoparticles of various sizes (50-450 nm), of different surface properties (OH, CO2H, NR4+, mNH2), and of different composition (silica, gold, and polystyrene) were synthesized and evaluated for biological activity toward skeletal cells. Osteoblast activity was most influenced by composition and size variables, whereas osteoclasts were most affected by surface property variation. The study also establishes nanoparticle mediated suppression of Nfatc1, a key transcriptional regulator for osteoclast differentiation, identifying a novel mechanism of action. Collectively, the study highlights how during the design of bioactive nanoparticles, it is vital to consider not only the myriad of physical properties that can be manipulated, but also that the characteristics of the target cell plays an equally integral role in determining biological outcome. STATEMENT OF SIGNIFICANCE: Silica nanomaterials represent a promising biomaterial for beneficial effects on bone mass and quality as well as regenerative tissue engineering and are currently being investigated for intrinsic bioactivity towards the primary cells responsible for skeletal homeostasis; osteoblasts and osteoclasts. The goal of the current study was to assess the physical properties of silica nanoparticles that impart intrinsic bioactivity by evaluating size, surface charge, and composition. Results reveal differential influences of the physical properties of nanoparticles towards osteoblasts and osteoclasts. This study provides new insights into the design of nanoparticles to specifically target different aspects of bone metabolism and highlights the opportunities provided by nanotechnology to modulate a range of cell specific biological responses for therapeutic benefit.
Keywords: Bone cells; Composition; Silica nanoparticles; Size; Surface charge.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

