Role of Host Cell Secretory Machinery in Zika Virus Life Cycle
- PMID: 30326556
- PMCID: PMC6213159
- DOI: 10.3390/v10100559
Role of Host Cell Secretory Machinery in Zika Virus Life Cycle
Abstract
The high human cost of Zika virus infections and the rapid establishment of virus circulation in novel areas, including the United States, present an urgent need for countermeasures against this emerging threat. The development of an effective vaccine against Zika virus may be problematic because of the cross reactivity of the antibodies with other flaviviruses leading to antibody-dependent enhancement of infection. Moreover, rapidly replicating positive strand RNA viruses, including Zika virus, generate large spectrum of mutant genomes (quasi species) every replication round, allowing rapid selection of variants resistant to drugs targeting virus-specific proteins. On the other hand, viruses are ultimate cellular parasites and rely on the host metabolism for every step of their life cycle, thus presenting an opportunity to manipulate host processes as an alternative approach to suppress virus replication and spread. Zika and other flaviviruses critically depend on the cellular secretory pathway, which transfers proteins and membranes from the ER through the Golgi to the plasma membrane, for virion assembly, maturation and release. In this review, we summarize the current knowledge of interactions of Zika and similar arthropod-borne flaviviruses with the cellular secretory machinery with a special emphasis on virus-specific changes of the secretory pathway. Identification of the regulatory networks and effector proteins required to accommodate the trafficking of virions, which represent a highly unusual cargo for the secretory pathway, may open an attractive and virtually untapped reservoir of alternative targets for the development of superior anti-viral drugs.
Keywords: Zika virus; flaviviruses; membrane trafficking; secretory pathway; virion maturation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

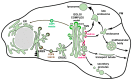
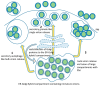
References
-
- Smithburn K.C., Kerr J.A., Gatne P.B. Neutralizing antibodies against certain viruses in the sera of residents of India. J. Immunol. 1954;72:248–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

