Investigation of Zirconium Effect on the Corrosion Resistance of Aluminum Alloy Using Electrochemical Methods and Numerical Simulation in an Acidified Synthetic Sea Salt Solution
- PMID: 30326579
- PMCID: PMC6213124
- DOI: 10.3390/ma11101982
Investigation of Zirconium Effect on the Corrosion Resistance of Aluminum Alloy Using Electrochemical Methods and Numerical Simulation in an Acidified Synthetic Sea Salt Solution
Abstract
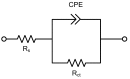
Corrosion resistance of Zr that has been added to an Al alloy (U1070) is higher than that of a commercial Al alloy (A1070). A decreasing number and size of Al₃Fe intermetallic particles (IMPs) were observed by electron microprobe analysis and transmission electron microscopy. Based on the numerical corrosion simulation, it was confirmed that decreasing the number and size of IMPs was favorable for improving the corrosion resistance of the Al alloy due to the reduction of the galvanic effect. In addition, Al₃Zr was found to be insignificant in promoting galvanic corrosion within the Al matrix. Thus, Zr is an advantageous alloying element for improving the corrosion resistance of the Al alloy.
Keywords: aluminum alloy; corrosion; intermetallic compound; transmission electron microscopy; zirconium.
Conflict of interest statement
Yong-Sang Kim and other co-authors have no conflict of interest.
Figures











References
-
- Davis J.R. Corrosion of Aluminum and Aluminum Alloys. ASM International; Materials Park, OH, USA: 1999.
-
- Akers W.W., Deans H.A., Crosser O.K. Condensation Heat Transfer within Horizontal Tubes. Chem. Eng. Prog. 1959;55:171–176.
-
- Akersm W.W., Rosson H.F. Condensation inside a horizontal tube. Chem. Eng. Prog. Symp. Ser. 1960;56:145.
-
- Cavallini A., Del Col D., Doretti L., Matkovic M., Rosseto L., Zilio C. Condensation heat transfer and pressure gradient inside multiple minichannels. Heat Transf. Eng. 2005;26:45–55. doi: 10.1080/01457630590907194. - DOI
-
- Adams T.M., Dowling M.F., Abdel-Khalik S.I., Jeter S.M. Applicability of traditional turbulent single phase forced convention correlations to non-circular microchannels. J. Heat Mass Transf. 1999;42:4411–4415. doi: 10.1016/S0017-9310(99)00102-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

