Temperature Sensitive Mutations in Influenza A Viral Ribonucleoprotein Complex Responsible for the Attenuation of the Live Attenuated Influenza Vaccine
- PMID: 30326610
- PMCID: PMC6213772
- DOI: 10.3390/v10100560
Temperature Sensitive Mutations in Influenza A Viral Ribonucleoprotein Complex Responsible for the Attenuation of the Live Attenuated Influenza Vaccine
Abstract
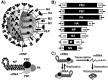
Live attenuated influenza vaccines (LAIV) have prevented morbidity and mortality associated with influenza viral infections for many years and represent the best therapeutic option to protect against influenza viral infections in humans. However, the development of LAIV has traditionally relied on empirical methods, such as the adaptation of viruses to replicate at low temperatures. These approaches require an extensive investment of time and resources before identifying potential vaccine candidates that can be safely implemented as LAIV to protect humans. In addition, the mechanism of attenuation of these vaccines is poorly understood in some cases. Importantly, LAIV are more efficacious than inactivated vaccines because their ability to mount efficient innate and adaptive humoral and cellular immune responses. Therefore, the design of potential LAIV based on known properties of viral proteins appears to be a highly appropriate option for the treatment of influenza viral infections. For that, the viral RNA synthesis machinery has been a research focus to identify key amino acid substitutions that can lead to viral attenuation and their use in safe, immunogenic, and protective LAIV. In this review, we discuss the potential to manipulate the influenza viral RNA-dependent RNA polymerase (RdRp) complex to generate attenuated forms of the virus that can be used as LAIV for the treatment of influenza viral infections, one of the current and most effective prophylactic options for the control of influenza in humans.
Keywords: attenuated; cold-adapted; influenza vaccine; influenza virus; live-attenuated influenza virus; nucleoprotein; recombinant influenza virus; temperature-sensitive; viral polymerase complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Foni E., Chiapponi C., Baioni L., Zanni I., Merenda M., Rosignoli C., Kyriakis C.S., Luini M.V., Mandola M.L., Bolzoni L., et al. Influenza D in Italy: Towards a better understanding of an emerging viral infection in swine. Sci. Rep. 2017;7:11660. doi: 10.1038/s41598-017-12012-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

