Exploring Protein⁻Protein Interaction in the Study of Hormone-Dependent Cancers
- PMID: 30326622
- PMCID: PMC6213999
- DOI: 10.3390/ijms19103173
Exploring Protein⁻Protein Interaction in the Study of Hormone-Dependent Cancers
Abstract
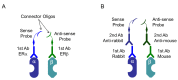
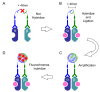
Estrogen receptors promote target gene transcription when they form a dimer, in which two identical (homodimer) or different (heterodimer) proteins are bound to each other. In hormone-dependent cancers, hormone receptor dimerization plays pivotal roles, not only in the pathogenesis or development of the tumors, but also in the development of therapeutic resistance. Protein⁻protein interactions (PPIs), including dimerization and complex formation, have been also well-known to be required for proteins to exert their functions. The methods which could detect PPIs are genetic engineering (i.e., resonance energy transfer) and/or antibody technology (i.e., co-immunoprecipitation) using cultured cells. In addition, visualization of the target proteins in tissues can be performed using antigen⁻antibody reactions, as in immunohistochemistry. Furthermore, development of microscopic techniques (i.e., electron microscopy and confocal laser microscopy) has made it possible to visualize intracellular and/or intranuclear organelles. We have recently reported the visualization of estrogen receptor dimers in breast cancer tissues by using the in situ proximity ligation assay (PLA). PLA was developed along the lines of antibody technology development, and this assay has made it possible to visualize PPIs in archival tissue specimens. Localization of PPI in organelles has also become possible using super-resolution microscopes exceeding the resolution limit of conventional microscopes. Therefore, in this review, we summarize the methodologies used for studying PPIs in both cells and tissues, and review the recently reported studies on PPIs of hormones.
Keywords: bioluminescence resonance energy transfer/förster resonance energy transfer; co-immunoprecipitation; estrogen receptor; immunohistochemistry; in situ proximity ligation assay; protein–protein interaction; super-resolution microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P.G., et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

