AFF3 upregulation mediates tamoxifen resistance in breast cancers
- PMID: 30326937
- PMCID: PMC6192118
- DOI: 10.1186/s13046-018-0928-7
AFF3 upregulation mediates tamoxifen resistance in breast cancers
Abstract
Background: Although tamoxifen is a highly effective drug for treating estrogen receptor-positive (ER+) breast cancer, nearly all patients with metastasis with initially responsive tumors eventually relapse, and die from acquired drug resistance. Unfortunately, few molecular mediators of tamoxifen resistance have been described. Here, we describe AFF3 (AF4/FMR2 family member 3), which encodes a nuclear protein with transactivation potential that confers tamoxifen resistance and enables estrogen-independent growth.
Methods: We investigated AFF3 expression in breast cancer cells and in clinical breast cancer specimens with western blot and Real-time PCR. We also examined the effects of AFF3 knockdown and overexpression on breast cancer cells using luciferase, tetrazolium, colony formation, and anchorage-independent growth assays in vitro and with nude mouse xenografting in vivo.
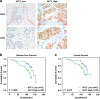
Results: AFF3 was overexpressed in tamoxifen-resistant tumors. AFF3 overexpression in breast cancer cells resulted in tamoxifen resistance, whereas RNA interference-mediated gene knockdown reversed this phenotype. Furthermore, AFF3 upregulation led to estrogen-independent growth in the xenograft assays. Mechanistic investigations revealed that AFF3 overexpression activated the ER signaling pathway and transcriptionally upregulated a subset of ER-regulated genes. Clinical analysis showed that increased AFF3 expression in ER+ breast tumors was associated with worse overall survival.
Conclusions: These studies establish AFF3 as a key mediator of estrogen-independent growth and tamoxifen resistance and as a potential novel diagnostic and therapeutic target.
Keywords: AFF3; Breast cancer; Estrogen receptor-positive; Resistance; Tamoxifen.
Conflict of interest statement
Ethics approval and consent to participate
For the use of clinical tissues for research purposes, the prior consent of the patients and approval from the Institutional Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University were obtained. All animal experiments were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Sun Yat-sen University.
Consent for publication
All authors approved of the manuscript and consented to its publication.
Competing interests
The authors declare that have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

