Visualizing hematopoiesis as a stochastic process
- PMID: 30327372
- PMCID: PMC6199667
- DOI: 10.1182/bloodadvances.2018023705
Visualizing hematopoiesis as a stochastic process
Abstract
Stochastic simulation has played an important role in understanding hematopoiesis, but implementing and interpreting mathematical models requires a strong statistical background, often preventing their use by many clinical and translational researchers. Here, we introduce a user-friendly graphical interface with capabilities for visualizing hematopoiesis as a stochastic process, applicable to a variety of mammal systems and experimental designs. We describe the visualization tool and underlying mathematical model, and then use this to simulate serial transplantations in mice, human cord blood cell expansion, and clonal hematopoiesis of indeterminate potential. The outcomes of these virtual experiments challenge previous assumptions and provide examples of the flexible range of hypotheses easily testable via the visualization tool.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
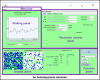
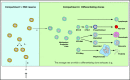
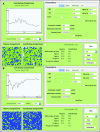



References
-
- Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96(10):3399-3405. - PubMed
-
- Abkowitz JL, Catlin SN, McCallie MT, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100(7):2665-2667. - PubMed

