Comprehensive elucidation of the structural and functional roles of engineered disulfide bonds in antibody Fc fragment
- PMID: 30327432
- PMCID: PMC6295723
- DOI: 10.1074/jbc.RA118.005367
Comprehensive elucidation of the structural and functional roles of engineered disulfide bonds in antibody Fc fragment
Abstract
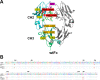
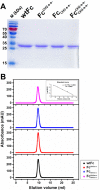
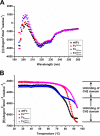
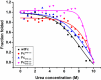
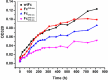
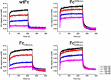
Therapeutic monoclonal antibodies and Fc-fusion proteins containing antibody Fc fragment may tend to destabilize (e.g. unfold and aggregate), which leads to loss of functions and increase of adverse risks. Although engineering of an additional disulfide bond has been performed in Fc or Fc domains for optimization, the relationships between introduced disulfide bond and alteration of the stability, aggregation propensity and function were still unclear and should be addressed for achievement of better therapeutic outcome. Here, we constructed three human IgG1 Fc mutants including FcCH2-s-s- (one engineered disulfide bond in CH2 domain), FcCH3-s-s- (one engineered disulfide bond in CH3 domain), and FcCH3-s-s-CH2-s-s- (two engineered disulfide bonds in CH2 and CH3 domains, respectively) for evaluation. As expected, each mutated domain shows obviously increased stability during thermo-induced unfolding, and FcCH3-s-s-CH2-s-s- is most thermo-stable among wildtype Fc (wtFc) and three mutants. The order of overall stability against denaturant is FcCH3-s-s-CH2-s-s- > FcCH2-s-s- > FcCH3-s-s- > wtFc. Then the aggregation propensity was compared among these four proteins. Under conditions of incubation at 60 °C, their aggregation resistance is in the order of FcCH3-s-s-CH2-s-s- > FcCH2-s-s- > FcCH3-s-s- ≈ wtFc. In contrast, the order is FcCH3-s-s-CH2-s-s- > FcCH3-s-s- > FcCH2-s-s- ≈ wtFc under acidic conditions. In addition, the Fc-mediated functions are not obviously affected by engineered disulfide bond. Our results give a comprehensive elucidation of structural and functional effects caused by additional disulfide bonds in the Fc fragment, which is important for Fc engineering toward the desired clinical performance.
Keywords: Fc engineering; Fc fragment; Fc receptor; aggregation; circular dichroism (CD); disulfide bond; function; fusion protein; monoclonal antibody; stability.
© 2018 Zeng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

