High levels of circulating GM-CSF+CD4+ T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study
- PMID: 30327490
- PMCID: PMC6804788
- DOI: 10.1038/s41423-018-0164-2
High levels of circulating GM-CSF+CD4+ T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study
Abstract
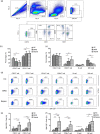
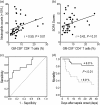
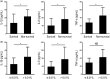
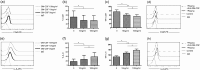
Granulocyte colony-stimulating factor (GM-CSF), produced by CD4+ T cells, has recently been implicated in the pathogenesis of inflammatory diseases, such as multiple sclerosis and juvenile arthritis. However, the role of GM-CSF-producing CD4+ T cells in sepsis remains unknown. This study reports peripheral changes in GM-CSF-producing CD4+ T cells in septic patients and the possible underlying mechanism by which GM-CSF influences the outcome of sepsis. Forty-three septic patients, 20 SIRS patients, and 20 healthy controls were enrolled in this study and followed for 28 days to assess mortality. We measured the peripheral frequency of GM-CSF+CD4+ T cells and recorded their associated relationship with disease progression. Our data demonstrated that peripheral GM-CSF-producing CD4+ T cells were significantly higher in septic patients than in both SIRS patients and healthy controls. These cells exhibit a memory phenotype and impaired IFN-γ-secreting capacity in sepsis patients. Using a receiver operating curve analysis with 8.01% as a cut-off point, the percentage of GM-CSF+CD4+ T cells could predict the outcome of septic patients. Combined with the increase in GM-CSF-producing CD4+ T cells, inflammatory cytokines IL-1β and IL-6 were also upregulated. Using an in vitro neutrophil model, we found that GM-CSF inhibited C3aR expression, while inducing IL-8 production. Furthermore, this effect was transferrable in plasma from sepsis patients and was attenuated by inhibition of GM-CSF using an anti-GM-CSF antibody. These results indicate that GM-CSF-producing CD4+ T cells may serve as a marker of sepsis severity. Thus, targeting GM-CSF overproduction may benefit sepsis patients.
Keywords: CD4+ T cells; GM-CSF; SIRS; sepsis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

