Novel Strategies for Peptide-Based Vaccines in Hematological Malignancies
- PMID: 30327655
- PMCID: PMC6174926
- DOI: 10.3389/fimmu.2018.02264
Novel Strategies for Peptide-Based Vaccines in Hematological Malignancies
Abstract
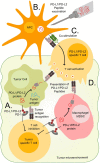
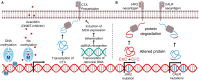
Peptides vaccination is an interesting approach to activate T-cells toward desired antigens in hematological malignancies. In addition to classical tumor associated antigens, such as cancer testis antigens, new potential targets for peptide vaccination comprise neo-antigens including JAK2 and CALR mutations, and antigens from immune regulatory proteins in the tumor microenvironment such as programmed death 1 ligands (PD-L1 and PD-L2). Immunosuppressive defenses of tumors are an important challenge to overcome and the T cell suppressive ligands PD-L1 and PD-L2 are often present in tumor microenvironments. Thus, PD-L1 and PD-L2 are interesting targets for peptide vaccines in diseases where the tumor microenvironment is known to play an essential role such as multiple myeloma and follicular lymphoma. In myelodysplastic syndromes the drug azacitidine re-exposes tumor associated antigens, why vaccination with related peptides would be an interesting addition. In myeloproliferative neoplasms the JAK2 and CALR mutations has proven to be immunogenic neo-antigens and thus possible targets for peptide vaccination. In this mini review we summarize the basis for these novel approaches, which has led to the initiation of clinical trials with various peptide vaccines in myelodysplastic syndromes, myeloproliferative neoplasms, multiple myeloma, and follicular lymphoma.
Keywords: PD-1; cancer testis antigen; follicular lymphoma; multiple myeloma; myelodysplastic syndrome; myeloproliferative neoplasms; neo-antigens; peptide vaccination.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

