Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea
- PMID: 30327839
- PMCID: PMC6349487
- DOI: 10.1007/s00018-018-2938-1
Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea
Abstract
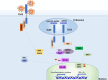
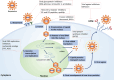
Herpes simplex virus type-1 (HSV-1) is a ubiquitous pathogen that infects a large majority of the human population worldwide. It is also a leading cause of infection-related blindness in the developed world. HSV-1 infection of the cornea begins with viral entry into resident cells via a multistep process that involves interaction of viral glycoproteins and host cell surface receptors. Once inside, HSV-1 infection induces a chronic immune-inflammatory response resulting in corneal scarring, thinning and neovascularization. This leads to development of various ocular diseases such as herpes stromal keratitis, resulting in visual impairment and eventual blindness. HSV-1 can also invade the central nervous system and lead to encephalitis, a relatively common cause of sporadic fetal encephalitis worldwide. In this review, we discuss the pathological processes activated by corneal HSV-1 infection and existing antiviral therapies as well as novel therapeutic options currently under development.
Keywords: Anti-viral therapy; Herpes simplex virus; Immune response; Ocular herpes; Pathological process.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

