Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry
- PMID: 30327931
- PMCID: PMC6244642
- DOI: 10.1007/s00415-018-9083-5
Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry
Abstract
Background: Comparative effectiveness (CE) research allows real-world treatment comparisons using outcome measurements important to physicians/patients. This German NeuroTransData registry-based analysis compared delayed-release dimethyl fumarate (DMF) effectiveness with interferons (IFN), glatiramer acetate (GA), teriflunomide (TERI), or fingolimod (FTY) in patients with relapsing-remitting multiple sclerosis (RRMS) using propensity score matching (PSM).
Methods: Data from registry patients aged ≥ 18 years with RRMS, ≥ 1 relapse, and Expanded Disability Status Scale (EDSS) assessment(s) after index therapy initiation underwent 1:1 PSM to match DMF with comparator populations baseline characteristics. Primary outcome measurement was time to first relapse (TTFR). Secondary outcome measurements included annualised relapse rate (ARR), proportion of patients relapse free at 12 and 24 months, time to index therapy discontinuation (TTD), and reasons for discontinuation. Exploratory analyses included time to 3- and 6-month EDSS confirmed disability progression (CDP). Non-pairwise censoring was the primary analysis method; pairwise censoring was the main sensitivity analysis method.
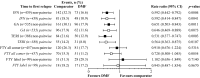
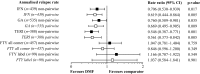
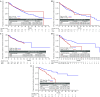
Findings: Post-matched cohorts were well-balanced. By non-pairwise censoring, TTFR and ARR were significantly lower in DMF populations versus matched IFN, GA, and TERI, but there was no evidence of difference between DMF and FTY. TTD was similar between DMF and IFN, GA, and TERI, but significantly shorter versus FTY. Time to CDP generally showed no evidence of difference between DMF and comparator populations. Pairwise censored analysis results confirmed the non-pairwise censoring results.
Interpretation: These results support previous CE studies in demonstrating relative improvement in real-world effectiveness with DMF versus first-line agents IFN, GA, and TERI, and similar effectiveness versus FTY.
Keywords: Comparative effectiveness research; Dimethyl fumarate; Multiple sclerosis; Observational data; Propensity score matching; Registry.
Conflict of interest statement
Conflicts of interest
SB received honoraria from Kassenärztliche Vereinigung Bayerns and health maintenance organisations for patient care, and from Biogen, MedDay, NeuroTransData, Novartis, and Roche for consulting, project management, clinical studies, and lectures; he also received honoraria and expense compensation as a board member of NeuroTransData. SG and PvH are employees of PwC, which was contracted to perform the statistical analysis for NeuroTransData. UF, RH, and FP are employees of and hold stock/stock options in Biogen. AB received honoraria from NeuroTransData for project management, clinical studies, and travel expenses from Novartis and Servier; he also received honoraria and expense compensation as a board member of NeuroTransData.
Ethical approval
The manuscript does not contain clinical studies or individually identifiable patient data.
Figures

References
-
- Chan A, Cutter G, Fox RJ, Xiao J, Lewin JB, Edwards MR. Comparative effectiveness of delayed-release dimethyl fumarate versus glatiramer acetate in multiple sclerosis patients: results of a matching-adjusted indirect comparison. J Comp Eff Res. 2017;6:313–323. doi: 10.2217/cer-2016-0085. - DOI - PubMed
-
- Fox R, Hutchinson M, Havrdova E, Kurukulasuriya N, Siddiqui K, Sarda S. Systematic review and mixed treatment comparison of delayed-release dimethyl fumarate and other disease-modifying therapies in treatment-naïve patients with relapsing-remitting multiple sclerosis. Neurology. 2015;84(14 suppl):P3.243.
-
- Fox RJ, Chan A, Zhang A, Xiao J, Levison D, Lewin JB, Edwards MR, Marantz JL. Comparative effectiveness using a matching-adjusted indirect comparison between delayed-release dimethyl fumarate and fingolimod for the treatment of multiple sclerosis. Curr Med Res Opin. 2017;33:175–183. doi: 10.1080/03007995.2016.1248380. - DOI - PubMed
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Investigators CS. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. - DOI - PubMed
-
- Hutchinson M, Fox RJ, Havrdova E, Kurukulasuriya NC, Sarda SP, Agarwal S, Siddiqui MK, Taneja A, Deniz B. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin. 2014;30:613–627. doi: 10.1185/03007995.2013.863755. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

