Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin
- PMID: 30328058
- PMCID: PMC6418071
- DOI: 10.1007/s13318-018-0517-3
Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin
Abstract
Background and objectives: The importance of quercetin and flavonoids in the diet and as food supplements is well known, and literature studies support their potential use to treat several human diseases. Many beneficial properties have been described for quercetin, so much effort has been directed into overcoming the major drawbacks of this natural compound-its poor solubility and low oral absorption. The aims of this study were to compare a new food-grade lecithin-based formulation of quercetin, Quercetin Phytosome®, to unformulated quercetin in terms of solubility in simulated gastrointestinal fluids and oral absorption in a randomized crossover pharmacokinetic study of healthy volunteers.
Methods: The solubility of the new formulation was determined by in vitro incubation in simulated gastrointestinal fluids, and quercetin was detected by ultra performance liquid chromatography. A single-dose, randomized, six-sequence/three-period crossover clinical trial (3 × 3 × 3 crossover design) with a balanced carryover effect was conducted in healthy volunteers under fasting conditions. Twelve healthy volunteers of both sexes with an age range of 18-50 years were recruited; one dose of quercetin and two different doses of Quercetin Phytosome were administered orally as film-coated tablets. Pharmacokinetic samples were collected at twelve time points (from 0 h to 24 h) after administration, and quercetin levels were measured by HPLC/MS/MS. Data were analyzed using the Phoenix WinNonlin (v.6.4) software package, and the most significant pharmacokinetic parameters were calculated. Statistical analysis involved performing a two-way ANOVA with repeated measures followed by post hoc analysis (Tukey's test).
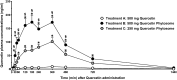
Results: Significant improvements in both in vitro solubility and oral absorption (in terms of both exposure and maximum concentration achieved) by healthy volunteers in a human clinical study were obtained with the Quercetin Phytosome formulation as compared to unformulated quercetin.
Conclusions: A more soluble formulation of quercetin based on lecithin, Quercetin Phytosome, has recently been developed, and was found to facilitate the attainment of very high plasma levels of quercetin-up to 20 times more than usually obtained following a dose of quercetin-when the novel formulation was administered orally in human volunteers, and it did not have any notable side effects. These results suggest that Quercetin Phytosome allows the oral administration of quercetin in a safe and bioavailable manner, thus facilitating the effective utilization of this natural compound to treat various human diseases.
Conflict of interest statement
Conflict of interest
The authors Antonella Riva, Massimo Ronchi, Giovanna Petrangolini, Stefania Bosisio, and Pietro Allegrini are employees of Indena SpA.
Ethical approval
All procedures in this study are in accordance with the 1964 Helsinki Declaration (and its amendments) and the criteria of the ethics committee that approved the study.
Informed consent
Written informed consent, as reviewed by the ethics committee, was obtained individually from all subjects who participated in the study.
Figures

References
-
- Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remón A, M’hiri N, García-Lobato P, et al. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013;2013:bat070. doi: 10.1093/database/bat070. - DOI - PMC - PubMed
-
- Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, et al. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol. 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004. - DOI
-
- Chopra M, Fitzsimons PE, Strain JJ, Thurnham DI, Howard AN. Nonalcoholic red wine extract and quercetin inhibit LDL oxidation without affecting plasma antioxidant vitamin and carotenoid concentrations. Clin Chem. 2000;46(8 Pt 1):1162–1170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

