Cost-Effectiveness Analysis of Bezlotoxumab Added to Standard of Care Versus Standard of Care Alone for the Prevention of Recurrent Clostridium difficile Infection in High-Risk Patients in Spain
- PMID: 30328061
- PMCID: PMC6223985
- DOI: 10.1007/s12325-018-0813-y
Cost-Effectiveness Analysis of Bezlotoxumab Added to Standard of Care Versus Standard of Care Alone for the Prevention of Recurrent Clostridium difficile Infection in High-Risk Patients in Spain
Abstract
Introduction: Clostridium difficile infection (CDI) is the major cause of infectious nosocomial diarrhoea and is associated with considerable morbidity, mortality and economic impact. Bezlotoxumab administered in combination with standard of care (SoC) antibiotic therapy prevents recurrent CDI. This study assessed the cost-effectiveness of bezlotoxumab added to SoC, compared to SoC alone, to prevent the recurrence of CDI in high-risk patients from the Spanish National Health System perspective.
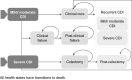
Methods: A Markov model was used to simulate the natural history of CDI over a lifetime horizon in five populations of patients at high risk of CDI recurrence according to MODIFY trials: (1) ≥ 65 years old; (2) severe CDI; (3) immunocompromised; (4) ≥ 1 CDI episode in the previous 6 months; and (5) ≥ 65 years old and with ≥ 1 CDI episode in the previous 6 months. The incremental cost-effectiveness ratio (ICER) expressed as cost per quality-adjusted life-year (QALY) gained was calculated. Deterministic (DSA) and probabilistic sensitivity analyses (PSA) were performed.
Results: In all patient populations (from 1 to 5), bezlotoxumab added to SoC reduced CDI recurrence compared to SoC alone by 26.4, 19.5, 21.2, 26.6 and 39.7%, respectively. The resulting ICERs for the respective subgroups were €12,724, €17,495, €9545, €7386, and €4378. The model parameters with highest impact on the ICER were recurrence rate (first), mortality, and utility values. The probability that bezlotoxumab was cost-effective at a willingness-to-pay threshold of €21,000/QALY was 85.5%, 54.1%, 86.0%, 94.5%, 99.6%, respectively.
Conclusion: The results suggest that bezlotoxumab added to SoC compared to SoC alone is a cost-effective treatment to prevent the recurrence of CDI in high-risk patients. The influence of changes in model parameters on DSA results was higher in patients ≥ 65 years old, with severe CDI and immunocompromised. Additionally, PSA estimated that the probability of cost-effectiveness exceeded 85% in most subgroups.
Funding: Merck Sharp & Dohme Corp.
Keywords: Bezlotoxumab; Clostridium difficile infection; Cost-effectiveness.
Conflict of interest statement
Miguel Salavert has received payment for consultancy services for the submitted work from MSD Spain. Miguel Salavert has served as speaker for Janssen; and has received payment for lectures from MSD Spain and Pfizer. Javier Cobo has received payment for consultancy services for the submitted work from MSD Spain. Javier Cobo has received payment for lectures and has participate in advisory boards for Astellas Pharma and MSD Spain. Javier Cobo has received payment for consultancy services for the submitted work from MSD Spain. Álvaro Pascual has received payment for consultancy services for the submitted work from MSD Spain. Álvaro Pascual has participate in advisory boards for MSD Spain. Santiago Grau has received payment for consultancy services for the submitted work from MSD Spain. Santiago Grau has served as a consultant for Angelini Farmacéutica, Astellas Pharma and MSD Spain. Belén Aragón is an employee of MSD Spain and may own stock or stock options in the Company. Yiling Jiang is an employee of Merck Sharp & Dohme Ltd. UK and may own stock or stock options in the Company. Stefano Maratia is an employee of Chiltern International Spain, a contract research organization providing support to MSD Spain. Susana Aceituno works for an independent research institution which has received fees for its contribution to the project coordination as well as to the drafting of this manuscript. The sponsor had no influence on the evaluation of data, the preparation of the manuscript and the decision to submit it for publication.
Figures



Similar articles
-
Cost-effectiveness of Bezlotoxumab Compared With Placebo for the Prevention of Recurrent Clostridium difficile Infection.Clin Infect Dis. 2018 Jan 18;66(3):355-362. doi: 10.1093/cid/cix809. Clin Infect Dis. 2018. PMID: 29106516 Clinical Trial.
-
Cost-effectiveness of three different strategies for the treatment of first recurrent Clostridium difficile infection diagnosed in a community setting.Infect Control Hosp Epidemiol. 2018 Aug;39(8):924-930. doi: 10.1017/ice.2018.139. Epub 2018 Jul 2. Infect Control Hosp Epidemiol. 2018. PMID: 29961435
-
Cost-effectiveness of bezlotoxumab and fidaxomicin for initial Clostridioides difficile infection.Clin Microbiol Infect. 2021 Oct;27(10):1448-1454. doi: 10.1016/j.cmi.2021.04.004. Epub 2021 Apr 17. Clin Microbiol Infect. 2021. PMID: 33878506 Free PMC article.
-
Cost-Effectiveness Analysis of REBYOTA™ (Fecal Microbiota, Live-jslm [FMBL]) Versus Standard of Care for the Prevention of Recurrent Clostridioides difficile Infection in the USA.Adv Ther. 2023 Jun;40(6):2784-2800. doi: 10.1007/s12325-023-02505-1. Epub 2023 Apr 24. Adv Ther. 2023. PMID: 37093359 Free PMC article.
-
Bezlotoxumab: A Review in Preventing Clostridium difficile Infection Recurrence.Drugs. 2017 Oct;77(15):1657-1663. doi: 10.1007/s40265-017-0809-y. Drugs. 2017. PMID: 28865041 Review.
Cited by
-
Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives.Gut Pathog. 2023 May 9;15(1):21. doi: 10.1186/s13099-023-00550-3. Gut Pathog. 2023. PMID: 37161478 Free PMC article. Review.
-
Is Three Company or a Crowd? Comparing and Contrasting U.S. and European Clostridioidesdifficile Clinical Practice Guidelines.Antibiotics (Basel). 2022 Sep 14;11(9):1247. doi: 10.3390/antibiotics11091247. Antibiotics (Basel). 2022. PMID: 36140026 Free PMC article. Review.
-
The Role of Bezlotoxumab for the Prevention of Recurrent Clostridioides difficile Infections: A Review of the Current Literature and Paradigm Shift after 2021.Antibiotics (Basel). 2022 Sep 7;11(9):1211. doi: 10.3390/antibiotics11091211. Antibiotics (Basel). 2022. PMID: 36139989 Free PMC article. Review.
-
Encapsulation protocol for fecal microbiota transplantation.Front Cell Infect Microbiol. 2024 Jun 26;14:1424376. doi: 10.3389/fcimb.2024.1424376. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38988813 Free PMC article.
-
Bezlotoxumab for Preventing Recurrent Clostridioides difficile Infection: A Narrative Review from Pathophysiology to Clinical Studies.Infect Dis Ther. 2020 Sep;9(3):481-494. doi: 10.1007/s40121-020-00314-5. Epub 2020 Jul 6. Infect Dis Ther. 2020. PMID: 32632582 Free PMC article. Review.
References
-
- Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis. 2014;14(12):1208–19. 10.1016/S1473-3099(14)70991-0 - DOI - PubMed
-
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. 10.1086/651706 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

