Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice
- PMID: 30328290
- PMCID: PMC6204589
- DOI: 10.1002/jcsm.12354
Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice
Erratum in
-
Corrigendum.J Cachexia Sarcopenia Muscle. 2019 Jun;10(3):712. doi: 10.1002/jcsm.12464. J Cachexia Sarcopenia Muscle. 2019. PMID: 31246374 Free PMC article. No abstract available.
Abstract
Background: Cancer cachexia occurs in approximately 80% of cancer patients and is a key contributor to cancer-related death. The mechanisms controlling development of tumour-induced muscle wasting are not fully elucidated. Specifically, the progression and development of cancer cachexia are underexplored. Therefore, we examined skeletal muscle protein turnover throughout the development of cancer cachexia in tumour-bearing mice.
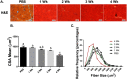
Methods: Lewis lung carcinoma (LLC) was injected into the hind flank of C57BL6/J mice at 8 weeks age with tumour allowed to develop for 1, 2, 3, or 4 weeks and compared with PBS injected control. Muscle size was measured by cross-sectional area analysis of haematoxylin and eosin stained tibialis anterior muscle. 2 H2 O was used to assess protein synthesis throughout the development of cancer cachexia. Immunoblot and RT-qPCR were used to measure regulators of protein turnover. TUNEL staining was utilized to measure apoptotic nuclei. LLC conditioned media (LCM) treatment of C2C12 myotubes was used to analyse cancer cachexia in vitro.
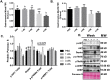
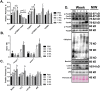
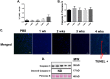
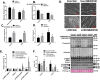
Results: Muscle cross-sectional area decreased ~40% 4 weeks following tumour implantation. Myogenic signalling was suppressed in tumour-bearing mice as soon as 1 week following tumour implantation, including lower mRNA contents of Pax7, MyoD, CyclinD1, and Myogenin, when compared with control animals. AchRδ and AchRε mRNA contents were down-regulated by ~50% 3 weeks following tumour implantation. Mixed fractional synthesis rate protein synthesis was ~40% lower in 4 week tumour-bearing mice when compared with PBS controls. Protein ubiquitination was elevated by ~50% 4 weeks after tumour implantation. Moreover, there was an increase in autophagy machinery after 4 weeks of tumour growth. Finally, ERK and p38 MAPK phosphorylations were fourfold and threefold greater than control muscle 4 weeks following tumour implantation, respectively. Inhibition of p38 MAPK, but not ERK MAPK, in vitro partially rescued LCM-induced loss of myotube diameter.
Conclusions: Our findings work towards understanding the pathophysiological signalling in skeletal muscle in the initial development of cancer cachexia. Shortly following the onset of the tumour-bearing state alterations in myogenic regulatory factors are apparent, suggesting early onset alterations in the capacity for myogenic induction. Cancer cachexia presents with a combination of a loss of protein synthesis and increased markers of protein breakdown, specifically in the ubiquitin-proteasome system. Also, p38 MAPK may be a potential therapeutic target to combat cancer cachexia via a p38-FOX01-atrogene-ubiquitin-proteasome mechanism.
Keywords: ERK; LLC; MAPK; Protein synthesis; Ubiquitin; p38.
© 2018 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures
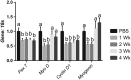
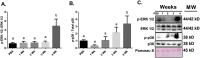

References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. - PubMed
-
- Fearon Kenneth CH, Glass David J, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

