Affinity profiling of monoclonal antibody and antibody-drug-conjugate preparations by coupled liquid chromatography-surface plasmon resonance biosensing
- PMID: 30328504
- PMCID: PMC6244757
- DOI: 10.1007/s00216-018-1414-y
Affinity profiling of monoclonal antibody and antibody-drug-conjugate preparations by coupled liquid chromatography-surface plasmon resonance biosensing
Abstract
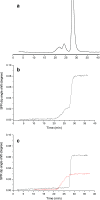
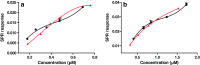
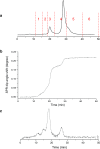
Monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) are highly potent biopharmaceuticals designed for targeted cancer therapies. mAbs and ADCs can undergo modifications during production and storage which may affect binding to target receptors, potentially altering drug efficacy. In this work, liquid chromatography was coupled online to surface plasmon resonance (LC-SPR) to allow label-free affinity evaluation of mAb and ADC sample constituents (size and charge variants), under near-native conditions. Trastuzumab and its ADC trastuzumab emtansine (T-DM1) were used as a test sample and were analyzed by aqueous size-exclusion chromatography (SEC)-SPR before and after exposure to aggregate-inducing conditions. SEC-SPR allowed separation of the formed aggregates and measurement of their affinity towards the ligand-binding domain of the human epidermal growth factor receptor 2 (HER2) receptor immobilized on the surface of the SPR sensor chip. The monomer and aggregates of the mAb and ADC were shown to have similar antigen affinity. Conjugation of drugs to trastuzumab appeared to accelerate the aggregate formation. In addition, cation-exchange chromatography (CEX) was coupled to SPR enabling monitoring the maximum ligand-analyte binding capacity (Rmax) of individual charge variants present in mAbs. Deamidated species and lysine variants in trastuzumab sample were separated but did not show different binding affinities to the immobilized HER2-binding domain. In order to allow protein variant assignment, parallel MS detection was added to the LC-SPR setup using a column effluent split. The feasibility of the LC-MS/SPR system was demonstrated by analysis of trastuzumab and T-DM1 providing information on antibody glycoforms and/or determination of the drug-to-antibody ratio (DAR), while simultaneously monitoring binding of eluting species to HER2. Graphical abstract ᅟ.
Keywords: Biopharmaceutical antibody-drug conjugates; Cation exchange chromatography; Online coupling; Size exclusion chromatography; Surface plasmon resonance; Trastuzumab.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Stability assessment of antibody-drug conjugate Trastuzumab emtansine in comparison to parent monoclonal antibody using orthogonal testing protocol.J Pharm Biomed Anal. 2018 Feb 20;150:268-277. doi: 10.1016/j.jpba.2017.12.022. Epub 2017 Dec 13. J Pharm Biomed Anal. 2018. PMID: 29258046
-
A Combination of Native LC-MS Approaches for the Comprehensive Characterization of the Antibody-Drug Conjugate Trastuzumab Deruxtecan.Front Biosci (Landmark Ed). 2022 Oct 26;27(10):290. doi: 10.31083/j.fbl2710290. Front Biosci (Landmark Ed). 2022. PMID: 36336868
-
Native mass spectrometry and ion mobility characterization of trastuzumab emtansine, a lysine-linked antibody drug conjugate.Protein Sci. 2015 Aug;24(8):1210-23. doi: 10.1002/pro.2666. Epub 2015 Mar 31. Protein Sci. 2015. PMID: 25694334 Free PMC article.
-
[Optical surface plasmon resonance biosensors in molecular fishing].Biomed Khim. 2015 Mar-Apr;61(2):231-8. doi: 10.18097/PBMC20156102231. Biomed Khim. 2015. PMID: 25978389 Review. Russian.
-
Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate.Ann Pharmacother. 2014 Nov;48(11):1484-93. doi: 10.1177/1060028014545354. Epub 2014 Jul 31. Ann Pharmacother. 2014. PMID: 25082874 Review.
Cited by
-
Glycan Profile Analysis of Engineered Trastuzumab with Rationally Added Glycosylation Sequons Presents Significantly Increased Glycan Complexity.Pharmaceutics. 2021 Oct 20;13(11):1747. doi: 10.3390/pharmaceutics13111747. Pharmaceutics. 2021. PMID: 34834161 Free PMC article.
-
Natural and Designed Toxins for Precise Therapy: Modern Approaches in Experimental Oncology.Int J Mol Sci. 2021 May 7;22(9):4975. doi: 10.3390/ijms22094975. Int J Mol Sci. 2021. PMID: 34067057 Free PMC article. Review.
-
First-in-Human, Phase 1 Dose-Escalation Study of Biparatopic Anti-HER2 Antibody-Drug Conjugate MEDI4276 in Patients with HER2-positive Advanced Breast or Gastric Cancer.Mol Cancer Ther. 2021 Aug;20(8):1442-1453. doi: 10.1158/1535-7163.MCT-20-0014. Epub 2021 May 27. Mol Cancer Ther. 2021. PMID: 34045233 Free PMC article. Clinical Trial.
-
Identification of critical chemical modifications by size exclusion chromatography of stressed antibody-target complexes with competitive binding.MAbs. 2021 Jan-Dec;13(1):1887612. doi: 10.1080/19420862.2021.1887612. MAbs. 2021. PMID: 33616001 Free PMC article.
-
Antibody and antibody fragments site-specific conjugation using new Q-tag substrate of bacterial transglutaminase.Cell Death Discov. 2024 Feb 15;10(1):79. doi: 10.1038/s41420-024-01845-3. Cell Death Discov. 2024. PMID: 38360912 Free PMC article.
References
-
- Hughes B. Monoclonal antibodies: expanding the mAb pool. Nat Rev Drug Discov. 2008;7:561. doi: 10.1038/nrd2625. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

