Analyzing Vaccine Trials in Epidemics With Mild and Asymptomatic Infection
- PMID: 30329134
- PMCID: PMC6357804
- DOI: 10.1093/aje/kwy239
Analyzing Vaccine Trials in Epidemics With Mild and Asymptomatic Infection
Abstract
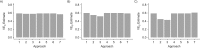
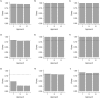
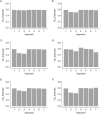
Vaccine efficacy against susceptibility to infection (VES), regardless of symptoms, is an important endpoint of vaccine trials for pathogens with a high proportion of asymptomatic infection, because such infections may contribute to onward transmission and long-term sequelae, such as congenital Zika syndrome. However, estimating VES is resource-intensive. We aimed to identify approaches for accurately estimating VES when limited information is available and resources are constrained. We modeled an individually randomized vaccine trial by generating a network of individuals and simulating an epidemic. The disease natural history followed a "susceptible-exposed-infectious/symptomatic (or infectious/asymptomatic)-recovered" model. We then used 7 approaches to estimate VES, and we also estimated vaccine efficacy against progression to symptoms (VEP). A corrected relative risk and an interval-censored Cox model accurately estimate VES and only require serological testing of participants once, while a Cox model using only symptomatic infections returns biased estimates. Only acquiring serological endpoints in a 10% sample and imputing the remaining infection statuses yields unbiased VES estimates across values of the basic reproduction number (R0) and accurate estimates of VEP for higher R0 values. Identifying resource-preserving methods for accurately estimating VES and VEP is important in designing trials for diseases with a high proportion of asymptomatic infection.
Figures

References
-
- World Health Organization List of Blueprint priority diseases. 2017. http://www.who.int/blueprint/priority-diseases/en/. Accessed October 9, 2018.
-
- Butler D. Billion-dollar project aims to prep vaccines before epidemics hit. Nature. 2017;541(7638):444–445. - PubMed
-
- Centers for Disease Control and Prevention Lassa fever. 2015. https://www.cdc.gov/vhf/lassa/index.html. Accessed October 9, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

