Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: Results of a multi-centre, randomised, placebo-controlled, double-blind, parallel-group trial (DoriPha)
- PMID: 30329199
- PMCID: PMC6282512
- DOI: 10.1111/ijcp.13272
Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: Results of a multi-centre, randomised, placebo-controlled, double-blind, parallel-group trial (DoriPha)
Abstract
Objective: The aim of this multi-centre, randomised, double-blind, placebo-controlled trial was to compare the efficacy and safety of the fixed combination of 0.5 mg tyrothricin, 1.0 mg benzalkonium chloride, and 1.5 mg benzocaine (study drug marketed as Dorithricin® ) in repeat dosing for 3 days to match placebo lozenges in the treatment of acute pharyngitis in adults.
Methods: Patients (pts, aged ≥18 years) with acute pharyngitis, ie, non-streptococcal sore throat and moderate-to-severe pain (intensity NRS ≥ 7; VAS ≥ 50) were assigned to study drug (n = 160) or matching placebo (n = 161). Efficacy was assessed by investigator for 2 hours post initial dose (p.i.d.), and 3 days later (Visit 2). Primary efficacy endpoint was the complete resolution of throat pain and difficulty in swallowing at Visit 2 (3 days p.i.d.). Safety and local tolerability were also assessed.
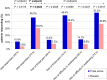
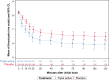
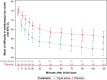
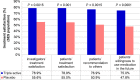
Results: Seventy-two hours (p.i.d.), complete resolution of throat pain and difficulty in swallowing were achieved by 44.6% patients on study drug compared with 27.2% patients on placebo (difference 17.4% (CI [5.8%; 29.7%]; 64% improvement [GEE, P = 0.0022]). Until 2 hours p.i.d., reduction in symptoms was better with study drug (P < 0.005). Treatment satisfaction was higher with study drug (patients'/investigators' assessment (78.9%/78.9% vs 55.0%/55.6% for placebo) and was well tolerated, overall safety profile was comparable to placebo.
Conclusion: The strength of this randomised controlled trial lies in the endpoint of complete remission after 3 days p.i.d., especially in the light of other trials addressing acute pharyngitis. The results of this study show a significant benefit of the study drug over placebo in the treatment of acute pharyngitis. Local treatment with the fixed combination (0.5 mg tyrothricin, 1.0 mg benzalkonium chloride, and 1.5 mg benzocaine) provides a rapid analgesic effect and is effective in relieving both severe throat pain as well as difficulty in swallowing associated with acute pharyngitis leading to a 64% improved complete remission within 72 hours. The triple active combination is a suitable treatment option for patients in the self-management of acute pharyngitis and sore throat.
Clinical trial registration: ClinicalTrials.gov, NCT03323528.
© 2018 The Authors. International Journal of Clinical Practice Published by John Wiley & Sons Ltd.
Figures

Comment in
-
Letter re: Article by Palm J et al Int J Clin Pract. 2018 Oct 17; 72:e13272.Int J Clin Pract. 2020 Feb;74(2):e13440. doi: 10.1111/ijcp.13440. Int J Clin Pract. 2020. PMID: 31633267 No abstract available.
References
-
- Sajjan US, Jia Y, Newcomb DC, Bentley JK, Lukacs NW, et al. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM‐1 and TLR3 expression. FASEB J. 2006;20(12):2121‐2123. - PubMed
-
- Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host‐microbiota mutualism. Nat Rev Microbiol. 2011;9:233‐243. - PubMed
-
- Planelles J. The pathogenic mechanism of superinfections in antibiotic therapy of bacterial infections. Z Arztl Fortbild (Jena). 1963;15(57):702‐707. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
