Periostin overexpression in collecting ducts accelerates renal cyst growth and fibrosis in polycystic kidney disease
- PMID: 30332313
- PMCID: PMC6336984
- DOI: 10.1152/ajprenal.00246.2018
Periostin overexpression in collecting ducts accelerates renal cyst growth and fibrosis in polycystic kidney disease
Abstract
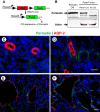
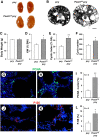
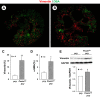
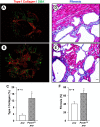
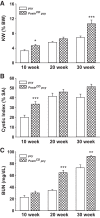
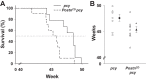
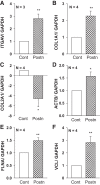
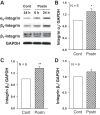
In polycystic kidney disease (PKD), persistent activation of cell proliferation and matrix production contributes to cyst growth and fibrosis, leading to progressive deterioration of renal function. Previously, we showed that periostin, a matricellular protein involved in tissue repair, is overexpressed by cystic epithelial cells of PKD kidneys. Periostin binds αVβ3-integrins and activates integrin-linked kinase (ILK), leading to Akt/mammalian target of rapamycin (mTOR)-mediated proliferation of human PKD cells. By contrast, periostin does not stimulate the proliferation of normal human kidney cells. This difference in the response to periostin is due to elevated expression of αVβ3-integrins by cystic cells. To determine whether periostin accelerates cyst growth and fibrosis, we generated mice with conditional overexpression of periostin in the collecting ducts (CDs). Ectopic CD expression of periostin was not sufficient to induce cyst formation or fibrosis in wild-type mice. However, periostin overexpression in pcy/pcy ( pcy) kidneys significantly increased mTOR activity, cell proliferation, cyst growth, and interstitial fibrosis; and accelerated the decline in renal function. Moreover, CD-specific overexpression of periostin caused a decrease in the survival of pcy mice. These pathological changes were accompanied by increased renal expression of vimentin, α-smooth muscle actin, and type I collagen. We also found that periostin increased gene expression of pathways involved in repair, including integrin and growth factor signaling and ECM production, and it stimulated focal adhesion kinase, Rho GTPase, cytoskeletal reorganization, and migration of PKD cells. These results suggest that periostin stimulates signaling pathways involved in an abnormal tissue repair process that contributes to cyst growth and fibrosis in PKD.
Keywords: autosomal dominant polycystic kidney disease; extracellular matrix; integrin; matricellular protein; proliferation.
Conflict of interest statement
D. P. Wallace is a consultant for Vertex Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures
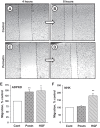
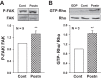
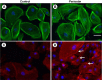
Comment in
-
Periostin and polycystic kidney disease: more pieces in the puzzle.Am J Physiol Renal Physiol. 2019 Jan 1;316(1):F159-F161. doi: 10.1152/ajprenal.00518.2018. Epub 2018 Nov 28. Am J Physiol Renal Physiol. 2019. PMID: 30484349 No abstract available.
References
-
- Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011. doi: 10.1681/ASN.2010050526. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

