Emerin induces nuclear breakage in Xenopus extract and early embryos
- PMID: 30332321
- PMCID: PMC6340207
- DOI: 10.1091/mbc.E18-05-0277
Emerin induces nuclear breakage in Xenopus extract and early embryos
Abstract
Emerin is an inner nuclear membrane protein often mutated in Emery-Dreifuss muscular dystrophy. Because emerin has diverse roles in nuclear mechanics, cytoskeletal organization, and gene expression, it has been difficult to elucidate its contribution to nuclear structure and disease pathology. In this study, we investigated emerin's impact on nuclei assembled in Xenopus laevis egg extract, a simplified biochemical system that lacks potentially confounding cellular factors and activities. Notably, these extracts are transcriptionally inert and lack endogenous emerin and filamentous actin. Strikingly, emerin caused rupture of egg extract nuclei, dependent on the application of shear force. In egg extract, emerin localized to nonnuclear cytoplasmic membranes, and nuclear rupture was rescued by targeting emerin to the nucleus, disrupting its membrane association, or assembling nuclei with lamin A. Furthermore, emerin induced breakage of nuclei in early-stage X. laevis embryo extracts, and embryos microinjected with emerin were inviable, with ruptured nuclei. We propose that cytoplasmic membrane localization of emerin leads to rupture of nuclei that are more sensitive to mechanical perturbation, findings that may be relevant to early development and certain laminopathies.
Figures
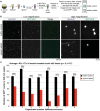
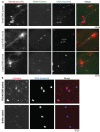
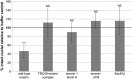

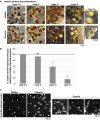

Similar articles
-
Emerin in health and disease.Semin Cell Dev Biol. 2014 May;29:95-106. doi: 10.1016/j.semcdb.2013.12.008. Epub 2013 Dec 21. Semin Cell Dev Biol. 2014. PMID: 24365856 Free PMC article. Review.
-
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells.J Cell Biol. 2005 Aug 29;170(5):781-91. doi: 10.1083/jcb.200502148. Epub 2005 Aug 22. J Cell Biol. 2005. PMID: 16115958 Free PMC article.
-
Expression and localization of nuclear proteins in autosomal-dominant Emery-Dreifuss muscular dystrophy with LMNA R377H mutation.BMC Cell Biol. 2004 Mar 30;5:12. doi: 10.1186/1471-2121-5-12. BMC Cell Biol. 2004. PMID: 15053843 Free PMC article.
-
The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype.J Cell Sci. 2002 Jan 15;115(Pt 2):341-54. doi: 10.1242/jcs.115.2.341. J Cell Sci. 2002. PMID: 11839786
-
The role of inner nuclear membrane protein emerin in myogenesis.FASEB J. 2025 Apr 15;39(7):e70514. doi: 10.1096/fj.202500323. FASEB J. 2025. PMID: 40178931 Free PMC article. Review.
Cited by
-
Overview of cellular homeostasis-associated nuclear envelope lamins and associated input signals.Front Cell Dev Biol. 2023 May 12;11:1173514. doi: 10.3389/fcell.2023.1173514. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37250905 Free PMC article. Review.
-
Nuclear Assembly in Giant Unilamellar Vesicles Encapsulating Xenopus Egg Extract.Small. 2025 Jun;21(25):e2412126. doi: 10.1002/smll.202412126. Epub 2025 May 20. Small. 2025. PMID: 40390663 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

