Aquablation among novice users in Canada: A WATER II subpopulation analysis
- PMID: 30332589
- PMCID: PMC6520060
- DOI: 10.5489/cuaj.5501
Aquablation among novice users in Canada: A WATER II subpopulation analysis
Abstract
Introduction: Surgical management options for lower urinary tract symptoms/benign prostatic hyperplasia (LUTS/BPH) associated with prostates of small to moderate volume (<80 cc) are numerous; however, for men with enlarged prostates (>80 cc), many of these options are neither safe nor effective. Recently, Aquablation (PROCEPT BioRobotics, Inc., U.S.), a precise ultrasound-guided, robotically executed prostatic ablative procedure, has become available with U.S. Food and Drug Administration approval. Herein, we present three-month safety and efficacy data from the cohort of Canadian men included in the WATER II trial.
Methods: WATER II is a prospective clinical trial of the Aquablation system for the treatment of BPH in men with prostate volumes between 80 and 150 cc. Only patients from Canada were included for this analysis. At baseline, subjects completed International Prostate Symptom Score (IPSS), as well as several validated questionnaires, uroflowmetry and post-void residual volume measurements, and underwent standard laboratory blood assessment. These were repeated at one and three months post-Aquablation.
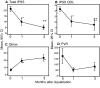
Results: A total of 19 subjects who met inclusion and exclusion criteria were enrolled at three Canadian academic sites. Mean pre-, one-month, and three-month post-treatment IPSS scores were 21.2±5.5, 9.9±6.9 (p<0.0001), and 5.0±4.5 (p<0.0001), respectively. Mean pre-, one-month, and three-month post-treatment maximum urinary flow rates (Qmax) were 6.6±3.1 ml/s, 19.5±6.1 ml/s (p<0.0001), and 23.1±9.2 ml/s (p<0.0001), respectively. The Clavien-Dindo grade 2 or higher event rate at three months was 31.6% (six events).
Conclusions: In this short-term, three-month analysis of Canadian men, Aquablation appears to provide a strong surgical alternative in patients with LUTS/BPH due to larger prostate volumes, with impressive functional outcomes, relatively short operative time and length of hospital stay, and acceptable complication and low transfusion rates.
Conflict of interest statement
Figures
References
-
- McVary KT. BPH: Epidemiology and comorbidities. Am J Manag Care. 2006;12:S122–8. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
