Dopamine Neurons Mediate Learning and Forgetting through Bidirectional Modulation of a Memory Trace
- PMID: 30332645
- PMCID: PMC6239218
- DOI: 10.1016/j.celrep.2018.09.051
Dopamine Neurons Mediate Learning and Forgetting through Bidirectional Modulation of a Memory Trace
Abstract
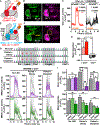
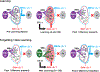
It remains unclear how memory engrams are altered by experience, such as new learning, to cause forgetting. Here, we report that short-term aversive memory in Drosophila is encoded by and retrieved from the mushroom body output neuron MBOn-γ2α'1. Pairing an odor with aversive electric shock creates a robust depression in the calcium response of MBOn-γ2α'1 and increases avoidance to the paired odor. Electric shock after learning, which activates the cognate dopamine neuron DAn-γ2α'1, restores the response properties of MBOn-γ2α'1 and causes behavioral forgetting. Conditioning with a second odor restores the responses of MBOn-γ2α'1 to a previously learned odor while depressing responses to the newly learned odor, showing that learning and forgetting can occur simultaneously. Moreover, optogenetic activation of DAn-γ2α'1 is sufficient for the bidirectional modulation of MBOn-γ2α'1 response properties. Thus, a single DAn can drive both learning and forgetting by bidirectionally modulating a cellular memory trace.
Keywords: dopamine neuron; forgetting; memory trace; memory updating.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

