Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration
- PMID: 30332775
- PMCID: PMC6222393
- DOI: 10.3390/molecules23102651
Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration
Abstract
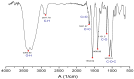
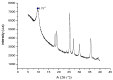
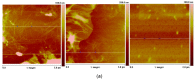
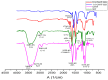
Several biomaterials, including natural polymers, are used to increase cellular interactions as an effective way to treat bone injuries. Chitosan (CS) is one of the most studied biocompatible natural polymers. Graphene oxide (GO) is a carbon-based nanomaterial capable of imparting desired properties to the scaffolds. In the present study, CS and GO were used for scaffold preparation. CS was extracted from the mycelium of the fungus Aspergillus niger. On the other hand, GO was synthesized using an improved Hummers-Offemann method and was characterized by Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, atomic force microscopy (AFM), X-ray diffraction (XRD), and dynamic light scattering (DLS). Subsequently, three formulations (GO 0%, 0.5%, and 1%) were used to prepare the scaffolds by the freeze-drying technique. The scaffolds were characterized by FTIR, thermogravimetric analysis (TGA), and scanning electron microscopy (SEM), to determine their thermal stability and pore size, demonstrating that their stability increased with the increase of GO amount. Finally, the scaffolds were implanted, recollected 30 days later, and studied with an optical microscope, which evidenced the recovery of the tissue architecture and excellent biocompatibility. Hence, these results strongly suggested the inherent nature of chitosan/graphene oxide (CS/GO) scaffolds for their application in bone tissue regeneration.
Keywords: chitosan; freeze-drying method; graphene oxide; scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Generation of graphene oxide and nano-bioglass based scaffold for bone tissue regeneration.Biomed Mater. 2022 Sep 30;17(6). doi: 10.1088/1748-605X/ac92b4. Biomed Mater. 2022. PMID: 36113451
-
Biological and structural properties of graphene oxide/curcumin nanocomposite incorporated chitosan as a scaffold for wound healing application.Life Sci. 2021 Jan 1;264:118640. doi: 10.1016/j.lfs.2020.118640. Epub 2020 Oct 24. Life Sci. 2021. PMID: 33172598
-
Graphene Oxide-A Tool for the Preparation of Chemically Crosslinking Free Alginate-Chitosan-Collagen Scaffolds for Bone Tissue Engineering.ACS Appl Mater Interfaces. 2018 Apr 18;10(15):12441-12452. doi: 10.1021/acsami.8b00699. Epub 2018 Apr 9. ACS Appl Mater Interfaces. 2018. PMID: 29589895
-
Role of crosslinkers in advancing chitosan-based biocomposite scaffolds for bone tissue engineering: A comprehensive review.Int J Biol Macromol. 2024 Dec;283(Pt 1):137625. doi: 10.1016/j.ijbiomac.2024.137625. Epub 2024 Nov 14. Int J Biol Macromol. 2024. PMID: 39547606 Review.
-
Unveiling the potential of biomaterials and their synergistic fusion in tissue engineering.Eur J Pharm Sci. 2024 May 1;196:106761. doi: 10.1016/j.ejps.2024.106761. Epub 2024 Apr 3. Eur J Pharm Sci. 2024. PMID: 38580169 Review.
Cited by
-
Biocompatible and Antimicrobial Electrospun Membranes Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide.Int J Mol Sci. 2019 Jun 19;20(12):2987. doi: 10.3390/ijms20122987. Int J Mol Sci. 2019. PMID: 31248075 Free PMC article.
-
Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes.Polymers (Basel). 2021 Nov 10;13(22):3877. doi: 10.3390/polym13223877. Polymers (Basel). 2021. PMID: 34833175 Free PMC article.
-
Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review.Molecules. 2023 Feb 17;28(4):1920. doi: 10.3390/molecules28041920. Molecules. 2023. PMID: 36838907 Free PMC article.
-
A Fast Adsorption of Azithromycin on Waste-Product-Derived Graphene Oxide Induced by H-Bonding and Electrostatic Interactions.ACS Omega. 2022 Aug 22;7(34):29655-29665. doi: 10.1021/acsomega.2c01919. eCollection 2022 Aug 30. ACS Omega. 2022. PMID: 36061663 Free PMC article.
-
Green Synthesized Gold Nanoparticles Using Viola betonicifolia Leaves Extract: Characterization, Antimicrobial, Antioxidant, and Cytobiocompatible Activities.Int J Nanomedicine. 2021 Oct 29;16:7319-7337. doi: 10.2147/IJN.S323524. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34754187 Free PMC article.
References
-
- Liu Y., Fang N., Liu B., Song L., Wen B., Yang D. Aligned Porous Chitosan/graphene Oxide Scaffold for Bone Tissue Engineering. Mater. Lett. 2018;233:78–81. doi: 10.1016/j.matlet.2018.08.108. - DOI
-
- Kerch G. Polymer Hydration and Stiffness at Biointerfaces and Related Cellular Processes. Nanomedicine-Nanotechnology. Biol. Med. 2018;14:13–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

