Recent Advances in the Catalytic Asymmetric Reactions of Oxaziridines
- PMID: 30332802
- PMCID: PMC6222879
- DOI: 10.3390/molecules23102656
Recent Advances in the Catalytic Asymmetric Reactions of Oxaziridines
Abstract
Oxaziridines have emerged as powerful and elegant oxygen- and nitrogen-transfer agents for a broad array of nucleophiles, due to the remarkably high and tunable reactivities. However, the asymmetric catalysis involving oxaziridines is still in its infancy. Herein, this review aims to examine recent advances in the catalytic asymmetric transformations of oxaziridines, including oxidation, amination, cycloaddition and deracemization.
Keywords: amination; aminohydroxylation; asymmetric catalysis; oxaziridines; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






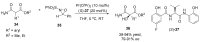

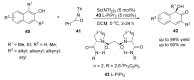
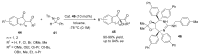


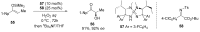


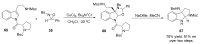
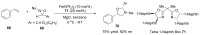



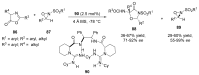

References
-
- Emmons W.D. The synthesis of oxaziridines. J. Am. Chem. Soc. 1956;78:6208–6209. doi: 10.1021/ja01604a072. - DOI
-
- Davis F.A., Chen B.-C. Asymmetric hydroxylation of enolates with N-sulfonyloxaziridines. Chem. Rev. 1992;92:919–934. doi: 10.1021/cr00013a008. - DOI
-
- Davis F.A., Reddy R.T., Han W., Reddy R.E. Asymmetric synthesis using N-sulfonyloxaziridines. Pure Appl. Chem. 1993;65:633–640. doi: 10.1351/pac199365040633. - DOI
-
- Davis F.A., Chen B.-C. Formation of C-O bonds by oxygenation of enolates. In: Helmchen G., Hoffman R.W., Mulzer J., Schaumann E., editors. Stereoselective Synthesis. Volume E21e. Georg Thieme Verlag; Stuttgart, Germany: 1995. p. 4497.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

