Resveratrol: Twenty Years of Growth, Development and Controversy
- PMID: 30332889
- PMCID: PMC6319551
- DOI: 10.4062/biomolther.2018.176
Resveratrol: Twenty Years of Growth, Development and Controversy
Abstract
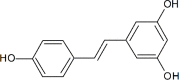
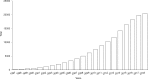
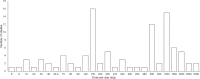
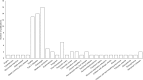
Resveratrol was first isolated in 1939 by Takaoka from Veratrum grandiflorum O. Loes. Following this discovery, sporadic descriptive reports appeared in the literature. However, spurred by our seminal paper published nearly 60 years later, resveratrol became a household word and the subject of extensive investigation. Now, in addition to appearing in over 20,000 research papers, resveratrol has inspired monographs, conferences, symposia, patents, chemical derivatives, etc. In addition, dietary supplements are marketed under various tradenames. Once resveratrol was brought to the limelight, early research tended to focus on pharmacological activities related to the cardiovascular system, inflammation, and cancer but, over the years, the horizon greatly expanded. Around 130 human clinical trials have been (or are being) conducted with varying results. This may be due to factors such as disparate doses (ca. 5 to 5,000 mg/day) and variable experimental settings. Further, molecular targets are numerous and a dominant mechanism is elusive or nonexistent. In this context, the compound is overtly promiscuous. Nonetheless, since the safety profile is pristine, and use as a dietary supplement is prevalent, these features are not viewed as detrimental. Given the ongoing history of resveratrol, it is reasonable to advocate for additional development and further clinical investigation. Topical preparations seem especially promising, as do conditions that can respond to anti-inflammatory action and/or direct exposure, such as colon cancer prevention. Although the ultimate fate of resveratrol remains an open question, thus far, the compound has inspired innovative scientific concepts and enhanced public awareness of preventative health care.
Keywords: Anti-inflammation; Clinical value; Discovery; Mechanism; Utilization.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Aggarwal BB, Shishodia S. S. Resveratrol in Health and Disease. Marcel Dekker, Inc.; New York: 2006.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

