Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania
- PMID: 30333022
- PMCID: PMC6192314
- DOI: 10.1186/s12936-018-2524-x
Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania
Abstract
Background: Artemisinin-based combination therapy (ACT) is the first-line anti-malarial treatment of uncomplicated malaria in most malaria endemic countries, including Tanzania. Unfortunately, there have been reports of artemisinin resistance and ACT failure from South East Asia highlighting the need to monitor therapeutic efficacy of ACT in these countries as recommended by World Health Organization.
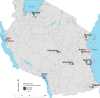
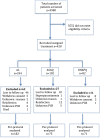
Methods: Open-label single arm studies in mainland Tanzania were conducted in nine sentinel sites in 2011, 2012 and 2015 to assess the efficacy and safety of artemether/lumefantrine (AL) and artesunate/amodiaquine (ASAQ) using 28 days follow-up and dihydroartemisinin/piperaquine (DHAPQ) using 42 days follow-up. Mutations in the propeller domain of the Plasmodium falciparum kelch 13 (k13) gene and amplification of the P. falciparum plasmepsin 2 (pm2) gene, associated with artemisinin and piperaquine (PQ) resistance, were also investigated.
Results: Of the 428 patients enrolled, 328 patients provided study endpoint. For AL, the PCR corrected per-protocol analysis showed adequate clinical and parasitological response (ACPR) of 90.3% (n = 28; 95% CI 74.2-98.0) in Kyela 2012, 95.7% (n = 22; 95% CI 78.1-99.0) in Chamwino, 100% in Muheza (n = 29; 95% CI 88.1-100), 100% in Nagaga (n = 39; 95% CI 91.0-100) and Kyela 2015 (n = 60; 95% CI 94.0-100). For ASAQ, PCR corrected ACPR of 98% (n = 49; 95% CI 89.4-99.9) and 100% (n = 25; 95% CI 86.3-100) were observed in 2011 in Ujiji and Kibaha, respectively. For DHAPQ, the ACPR was 100% (n = 71; 95% CI 94.9-100). Of the 235 samples with genetic interpretable results, only 7 (3%) had non-synonymous k13 mutations. None of these are candidate or validated markers of artemisinin resistance and all patients carrying these alleles cleared the parasites on day 3. Of the DHAPQ group, 10% (3/29) of the samples with interpretable results had pm2 multiple copies and none of them was associated with treatment failure.
Conclusion: All the tested ACT in mainland Tanzania were highly efficacious and none of validated k13 mutants associated with artemisinin resistance was observed. However, three isolates with multiple copy numbers of pm2 gene associated with PQ resistance among the limited samples tested successfully calls for further investigation. Trial registration Number ACTRN12615000159550. Registered 18th February 2015, https://www.anzctr.org.au/trial/MyTrial.aspx.
Keywords: Artemisinin; Artemisinin-based combination therapy; Efficacy; Malaria; Molecular markers; Piperaquine; Safety; Tanzania.
Figures
Similar articles
-
High cure rates and tolerability of artesunate-amodiaquine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Kibaha and Kigoma, Tanzania.Malar J. 2019 Mar 25;18(1):99. doi: 10.1186/s12936-019-2740-z. Malar J. 2019. PMID: 30909922 Free PMC article.
-
Efficacy and safety of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017.Malar J. 2018 Apr 3;17(1):144. doi: 10.1186/s12936-018-2290-9. Malar J. 2018. PMID: 29615039 Free PMC article.
-
Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial.Lancet. 2020 Apr 25;395(10233):1345-1360. doi: 10.1016/S0140-6736(20)30552-3. Epub 2020 Mar 11. Lancet. 2020. PMID: 32171078 Free PMC article. Clinical Trial.
-
Efficacy and safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ugandan children: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Apr 1;20(1):174. doi: 10.1186/s12936-021-03711-4. Malar J. 2021. PMID: 33794897 Free PMC article.
-
Paediatric formulations of artemisinin-based combination therapies for treating uncomplicated malaria in children.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD009568. doi: 10.1002/14651858.CD009568.pub2. Cochrane Database Syst Rev. 2020. PMID: 33289099 Free PMC article.
Cited by
-
A single low dose of primaquine is safe and sufficient to reduce transmission of Plasmodium falciparum gametocytes regardless of cytochrome P450 2D6 enzyme activity in Bagamoyo district, Tanzania.Malar J. 2022 Mar 12;21(1):84. doi: 10.1186/s12936-022-04100-1. Malar J. 2022. PMID: 35279143 Free PMC article. Clinical Trial.
-
The Diversity of the Plasmodium falciparum K13 Propeller Domain Did Not Increase after Implementation of Artemisinin-Based Combination Therapy in Uganda.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01234-19. doi: 10.1128/AAC.01234-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31358588 Free PMC article.
-
Baseline Ex Vivo and Molecular Responses of Plasmodium falciparum Isolates to Piperaquine before Implementation of Dihydroartemisinin-Piperaquine in Senegal.Antimicrob Agents Chemother. 2019 Apr 25;63(5):e02445-18. doi: 10.1128/AAC.02445-18. Print 2019 May. Antimicrob Agents Chemother. 2019. PMID: 30782997 Free PMC article.
-
Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions - India and sub-Saharan Africa.Int J Parasitol Drugs Drug Resist. 2021 Apr;15:43-56. doi: 10.1016/j.ijpddr.2020.11.006. Epub 2020 Dec 13. Int J Parasitol Drugs Drug Resist. 2021. PMID: 33556786 Free PMC article.
-
Efficacy of artesunate-amodiaquine for treatment of uncomplicated Plasmodium falciparum malaria in mainland Tanzania.Malar J. 2024 Mar 29;23(1):90. doi: 10.1186/s12936-024-04923-0. Malar J. 2024. PMID: 38553737 Free PMC article.
References
-
- WHO . World malaria report 2017. Geneva: World Health Organization; 2017.
-
- MOH. Tanzania Ministry of Health and Social Welfare. National guidelines for diagnosis and treatment of malaria; 2006.
-
- Leang R, Taylor WRJ, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin–piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. - DOI - PMC - PubMed
-
- WHO . Global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva: World Health Organization; 2010.
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials