Efficacy and safety of programmable compared with fixed anti-siphon devices for treating idiopathic normal-pressure hydrocephalus (iNPH) in adults - SYGRAVA: study protocol for a randomized trial
- PMID: 30333067
- PMCID: PMC6192316
- DOI: 10.1186/s13063-018-2951-6
Efficacy and safety of programmable compared with fixed anti-siphon devices for treating idiopathic normal-pressure hydrocephalus (iNPH) in adults - SYGRAVA: study protocol for a randomized trial
Abstract
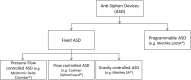
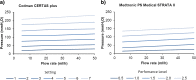
Background: Idiopathic normal-pressure hydrocephalus (iNPH) is a distinct form of dementia, characterized by gait ataxia, cognitive impairment and urinary incontinence. In contrast to all other causes of dementia (e.g., Alzheimer-type and others), ventriculoperitoneal (VP) shunt surgery may offer a curative treatment option to patients. While being a rather low-risk type of surgery, it may cause significant over- or underdrainage complications (e.g., headaches, dizziness, vomiting, intracerebral bleeding, etc.) during posture change. Anti-siphon devices (ASDs) are a group of technically different additional valves used in shunt surgery. They are designed to maintain intraventricular pressure within a normal physiological range regardless of patient position. Fixed ASDs proved to substantially lower the rate of overdrainage complications. No significant differences, however, were noted regarding underdrainage complications. Technical successors of fixed ASDs are programmable ASDs. The aim of this study is to evaluate whether programmable ASDs compared to fixed ASDs are able to avoid both over- and underdrainage complications.
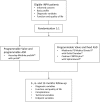
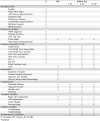
Methods/design: In this investigator-initiated, multicenter randomized trial, 306 patients are planned to be recruited. Male and female patients aged ≥18 years with iNPH who are eligible for VP shunt surgery and meet all other entry criteria can participate. Patients will be randomized in a balanced 1: 1 fashion to a VP shunt with a programmable valve either supplemented with a fixed ASD, or a programmable ASD. Patients will be followed-up 3, 6 and, on an optional basis, 12 months after surgery. The primary outcome measure is the cumulative incidence of over- or underdrainage 6 months post surgery, as defined by clinical and imaging parameters.
Discussion: SYGRAVA is the first randomized trial to determine whether programmable ASDs reduce complications of drainage compared to fixed ASDs in patients with iNPH. The results of this study may contribute to health-technology assessment of different valve systems used for VP-shunt surgery, and determination of the future standard of care.
Trial registration: International Standard Randomised Controlled Trial Number: ISRCTN13838310 . Registered on 10 November 2016.
Keywords: Dementia; Idiopathic normal-pressure hydrocephalus; Programmable anti-siphon device; Randomized trial; Ventriculoperitoneal shunt.
Conflict of interest statement
Ethics approval and consent to participate
The conduct of this study will comply with the International Conference for Harmonization Good Clinical Practice (ICH-GCP) regulations and guidelines and the current revision of the Declaration of Helsinki. Approval was obtained from the Charité Ethics Board on 2 September 2016 (EA1/234/16). Informed consent will be obtained from all participants before inclusion in the study. Participants may withdraw consent at any time.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

