Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics
- PMID: 30333213
- PMCID: PMC6240315
- DOI: 10.1242/dev.166728
Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics
Erratum in
-
Correction: Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics (doi: 10.1242/dev.166728).Development. 2019 Jan 22;146(2):dev175620. doi: 10.1242/dev.175620. Development. 2019. PMID: 30670566 Free PMC article. No abstract available.
-
Correction: Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics.Development. 2020 Sep 8;147(17):dev195610. doi: 10.1242/dev.195610. Development. 2020. PMID: 32900783 Free PMC article. No abstract available.
Abstract
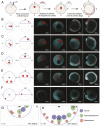
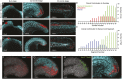
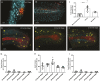
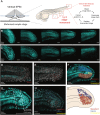
During gastrulation, embryonic cells become specified into distinct germ layers. In mouse, this continues throughout somitogenesis from a population of bipotent stem cells called neuromesodermal progenitors (NMps). However, the degree of self-renewal associated with NMps in the fast-developing zebrafish embryo is unclear. Using a genetic clone-tracing method, we labelled early embryonic progenitors and found a strong clonal similarity between spinal cord and mesoderm tissues. We followed individual cell lineages using light-sheet imaging, revealing a common neuromesodermal lineage contribution to a subset of spinal cord tissue across the anterior-posterior body axis. An initial population subdivides at mid-gastrula stages and is directly allocated to neural and mesodermal compartments during gastrulation. A second population in the tailbud undergoes delayed allocation to contribute to the neural and mesodermal compartment only at late somitogenesis. Cell tracking and retrospective cell fate assignment at late somitogenesis stages reveal these cells to be a collection of mono-fated progenitors. Our results suggest that NMps are a conserved population of bipotential progenitors, the lineage of which varies in a species-specific manner due to vastly different rates of differentiation and growth.
Keywords: Axial elongation; Gastrulation; Tailbud; Zebrafish.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

