Polyamine catabolism and oxidative damage
- PMID: 30333229
- PMCID: PMC6290137
- DOI: 10.1074/jbc.TM118.003337
Polyamine catabolism and oxidative damage
Abstract
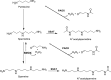
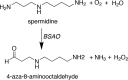
Polyamines (PAs) are indispensable polycations ubiquitous to all living cells. Among their many critical functions, PAs contribute to the oxidative balance of the cell. Beginning with studies by the Tabor laboratory in bacteria and yeast, the requirement for PAs as protectors against oxygen radical-mediated damage has been well established in many organisms, including mammals. However, PAs also serve as substrates for oxidation reactions that produce hydrogen peroxide (H2O2) both intra- and extracellularly. As intracellular concentrations of PAs can reach millimolar concentrations, the H2O2 amounts produced through their catabolism, coupled with a reduction in protective PAs, are sufficient to cause the oxidative damage associated with many pathologies, including cancer. Thus, the maintenance of intracellular polyamine homeostasis may ultimately contribute to the maintenance of oxidative homeostasis. Again, pioneering studies by Tabor and colleagues led the way in first identifying spermine oxidase in Saccharomyces cerevisiae. They also first purified the extracellular bovine serum amine oxidase and elucidated the products of its oxidation of primary amine groups of PAs when included in culture medium. These investigations formed the foundation for many polyamine-related studies and experimental procedures still performed today. This Minireview will summarize key innovative studies regarding PAs and oxidative damage, starting with those from the Tabor laboratory and including the most recent advances, with a focus on mammalian systems.
Keywords: antioxidant; cancer biology; free radicals; homeostasis; oxidase; oxidative stress; polyamine; polyamine catabolism; reactive oxygen species (ROS); redox regulation; spermidine; spermine.
© 2018 Murray Stewart et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
Nanoparticle strategies for cancer therapeutics: Nucleic acids, polyamines, bovine serum amine oxidase and iron oxide nanoparticles (Review).Int J Oncol. 2015 Jan;46(1):5-16. doi: 10.3892/ijo.2014.2706. Epub 2014 Oct 14. Int J Oncol. 2015. PMID: 25333509 Review.
-
The biological functions of polyamine oxidation products by amine oxidases: perspectives of clinical applications.Amino Acids. 2004 Dec;27(3-4):347-58. doi: 10.1007/s00726-004-0114-4. Epub 2004 Aug 19. Amino Acids. 2004. PMID: 15592759 Review.
-
Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants.Plant Physiol. 2004 Apr;134(4):1414-26. doi: 10.1104/pp.103.036764. Epub 2004 Apr 2. Plant Physiol. 2004. PMID: 15064377 Free PMC article.
-
Evaluation of the significance of polyamines and their oxidases in the aetiology of human cervical carcinoma.Br J Cancer. 1995 Nov;72(5):1194-9. doi: 10.1038/bjc.1995.485. Br J Cancer. 1995. PMID: 7577467 Free PMC article.
-
Catabolism of polyamines.Amino Acids. 2004 Jun;26(3):217-33. doi: 10.1007/s00726-004-0070-z. Epub 2004 Apr 20. Amino Acids. 2004. PMID: 15221502 Review.
Cited by
-
Spermidine supplementation influence on protective enzymes of Apis mellifera (Hymenoptera: Apidae).J Insect Sci. 2024 Sep 1;24(5):3. doi: 10.1093/jisesa/ieae098. J Insect Sci. 2024. PMID: 39382172 Free PMC article.
-
Overexpression of a S-Adenosylmethionine Decarboxylase from Sugar Beet M14 Increased Araidopsis Salt Tolerance.Int J Mol Sci. 2019 Apr 23;20(8):1990. doi: 10.3390/ijms20081990. Int J Mol Sci. 2019. PMID: 31018555 Free PMC article.
-
Transient Receptor Potential Ankyrin 1 (TRPA1) Channel Mediates Acrolein Cytotoxicity in Human Lung Cancer Cells.Int J Mol Sci. 2023 Jul 24;24(14):11847. doi: 10.3390/ijms241411847. Int J Mol Sci. 2023. PMID: 37511605 Free PMC article.
-
Protective effects of Scoparia dulcis L. extract on high glucose-induced injury in human retinal pigment epithelial cells.Front Nutr. 2023 Mar 30;10:1085248. doi: 10.3389/fnut.2023.1085248. eCollection 2023. Front Nutr. 2023. PMID: 37139437 Free PMC article.
-
Regulation of polyamine interconversion enzymes affects α-Synuclein levels and toxicity in a Drosophila model of Parkinson's Disease.NPJ Parkinsons Dis. 2025 Aug 6;11(1):231. doi: 10.1038/s41531-025-01087-9. NPJ Parkinsons Dis. 2025. PMID: 40770267 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

