Polyamines and potassium channels: A 25-year romance
- PMID: 30333230
- PMCID: PMC6290165
- DOI: 10.1074/jbc.TM118.003344
Polyamines and potassium channels: A 25-year romance
Abstract
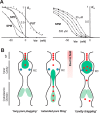
Potassium channels that exhibit the property of inward rectification (Kir channels) are present in most cells. Cloning of the first Kir channel genes 25 years ago led to recognition that inward rectification is a consequence of voltage-dependent block by cytoplasmic polyamines, which are also ubiquitously present in animal cells. Upon cellular depolarization, these polycationic metabolites enter the Kir channel pore from the intracellular side, blocking the movement of K+ ions through the channel. As a consequence, high K+ conductance at rest can provide very stable negative resting potentials, but polyamine-mediated blockade at depolarized potentials ensures, for instance, the long plateau phase of the cardiac action potential, an essential feature for a stable cardiac rhythm. Despite much investigation of the polyamine block, where exactly polyamines get to within the Kir channel pore and how the steep voltage dependence arises remain unclear. This Minireview will summarize current understanding of the relevance and molecular mechanisms of polyamine block and offer some ideas to try to help resolve the fundamental issue of the voltage dependence of polyamine block.
Keywords: ion channel; polyamine; potassium channel; protein structure; spermidine.
© 2018 Nichols and Lee.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Hille B. (1992) Ionic Channels of Excitable Membranes, Sinauer Associates, Sunderland, MA
-
- Katz B. (1949) Les constantes electriques de la membrane du muscle. Arch. Sci. Physiol. 2, 285–299
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

