A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans
- PMID: 30333238
- PMCID: PMC6330016
- DOI: 10.1126/scitranslmed.aat0797
A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans
Abstract
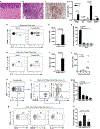
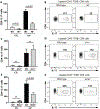
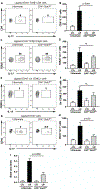
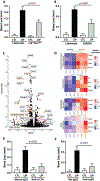
Periodontitis is one of the most common human inflammatory diseases, yet the mechanisms that drive immunopathology and could be therapeutically targeted are not well defined. Here, we demonstrate an expansion of resident memory T helper 17 (TH17) cells in human periodontitis. Phenocopying humans, TH17 cells expanded in murine experimental periodontitis through local proliferation. Unlike homeostatic oral TH17 cells, which accumulate in a commensal-independent and interleukin-6 (IL-6)-dependent manner, periodontitis-associated expansion of TH17 cells was dependent on the local dysbiotic microbiome and required both IL-6 and IL-23. TH17 cells and associated neutrophil accumulation were necessary for inflammatory tissue destruction in experimental periodontitis. Genetic or pharmacological inhibition of TH17 cell differentiation conferred protection from immunopathology. Studies in a unique patient population with a genetic defect in TH17 cell differentiation established human relevance for our murine experimental studies. In the oral cavity, human TH17 cell defects were associated with diminished periodontal inflammation and bone loss, despite increased prevalence of recurrent oral fungal infections. Our study highlights distinct functions of TH17 cells in oral immunity and inflammation and paves the way to a new targeted therapeutic approach for the treatment of periodontitis.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Cardoso CR et al., Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral microbiology and immunology 24, 1–6 (2009). - PubMed
-
- Allam JP et al., IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. Journal of clinical periodontology 38, 879–886 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

