Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models
- PMID: 30333311
- PMCID: PMC6237457
- DOI: 10.1172/jci.insight.121717
Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models
Abstract
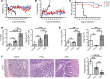
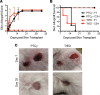
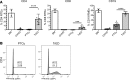
Posttransplant cyclophosphamide (PTCy) has been found to be effective in ameliorating acute graft-versus-host disease (GVHD) in patients following allogeneic hematopoietic stem cell transplantation (aHSCT). Adoptive transfer of high numbers of donor Tregs in experimental aHSCT has shown promise as a therapeutic modality for GVHD regulation. We recently described a strategy for in vivo Treg expansion targeting two receptors: TNFRSF25 and CD25. To date, there have been no direct comparisons between the use of PTCy and Tregs regarding outcome and immune reconstitution within identical groups of transplanted mice. Here, we assessed these two strategies and found both decreased clinical GVHD and improved survival long term. However, recipients transplanted with Treg-expanded donor cells (TrED) exhibited less weight loss early after HSCT. Additionally, TrED recipients demonstrated less thymic damage, significantly more recent thymic emigrants, and more rapid lymphoid engraftment. Three months after HSCT, PTCy-treated and TrED recipients showed tolerance to F1 skin allografts and comparable immune function. Overall, TrED was found superior to PTCy with regard to weight loss early after transplant and initial lymphoid engraftment. Based on these findings, we speculate that morbidity and mortality after transplant could be diminished following TrED transplant into aHSCT recipients, and, therefore, that TrED could provide a promising clinical strategy for GVHD prophylaxis.
Keywords: Bone marrow transplantation; Immunology; T cells; Transplantation.
Conflict of interest statement
Figures