Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer
- PMID: 30333318
- PMCID: PMC6237485
- DOI: 10.1172/jci.insight.122857
Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer
Abstract
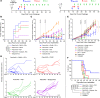
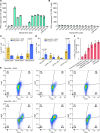
Tumor neoantigens arising from somatic mutations in the cancer genome are less likely to be subject to central immune tolerance and are therefore attractive targets for vaccine immunotherapy. We utilized whole-exome sequencing, RNA sequencing (RNASeq), and an in silico immunogenicity prediction algorithm, NetMHC, to generate a neoantigen-targeted vaccine, PancVAX, which was administered together with the STING adjuvant ADU-V16 to mice bearing pancreatic adenocarcinoma (Panc02) cells. PancVAX activated a neoepitope-specific T cell repertoire within the tumor and caused transient tumor regression. When given in combination with two checkpoint modulators, namely anti-PD-1 and agonist OX40 antibodies, PancVAX resulted in enhanced and more durable tumor regression and a survival benefit. The addition of OX40 to vaccine reduced the coexpression of T cell exhaustion markers, Lag3 and PD-1, and resulted in rejection of tumors upon contralateral rechallenge, suggesting the induction of T cell memory. Together, these data provide the framework for testing personalized neoantigen-based combinatorial vaccine strategies in patients with pancreatic and other nonimmunogenic cancers.
Keywords: Cancer immunotherapy; Oncology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

