Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation
- PMID: 30333323
- PMCID: PMC6237472
- DOI: 10.1172/jci.insight.121521
Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation
Abstract
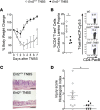
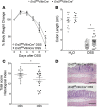
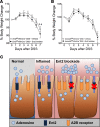
Intestinal epithelial barrier repair is vital for remission in inflammatory bowel disease (IBD). Extracellular adenosine signaling has been implicated in promoting restoration of epithelial barrier function. Currently, no clinically approved agents target this pathway. Adenosine signaling is terminated by uptake from the extracellular space via equilibrative nucleoside transporters (ENTs). We hypothesized that ENT inhibition could dampen intestinal inflammation. Initial studies demonstrated transcriptional repression of ENT1 and ENT2 in IBD biopsies or in murine IBD models. Subsequent studies in mice with global Ent1 or Ent2 deletion revealed selective protection of Ent2-/- mice. Elevated intestinal adenosine levels in conjunction with abolished protection following pharmacologic blockade of A2B adenosine receptors implicate adenosine signaling as the mechanism of gut protection in Ent2-/- mice. Additional studies in mice with tissue-specific deletion of Ent2 uncovered epithelial Ent2 as the target. Moreover, intestinal protection provided by a selective Ent2 inhibitor was abolished in mice with epithelium-specific deletion of Ent2 or the A2B adenosine receptor. Taken together, these findings indicate that increased mucosal A2B signaling following repression or deletion of epithelial Ent2 coordinates the resolution of intestinal inflammation. This study suggests the presence of a targetable purinergic network within the intestinal epithelium designed to limit tissue inflammation.
Keywords: Gastroenterology; Inflammatory bowel disease.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

