Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain
- PMID: 30333324
- PMCID: PMC6237481
- DOI: 10.1172/jci.insight.120922
Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain
Erratum in
-
Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain.JCI Insight. 2018 Dec 6;3(23):e126138. doi: 10.1172/jci.insight.126138. JCI Insight. 2018. PMID: 30518698 Free PMC article. No abstract available.
Abstract
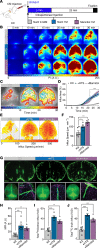
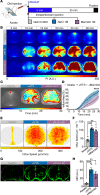
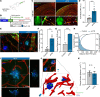
Despite the initial promise of immunotherapy for CNS disease, multiple recent clinical trials have failed. This may be due in part to characteristically low penetration of antibodies to cerebrospinal fluid (CSF) and brain parenchyma, resulting in poor target engagement. We here utilized transcranial macroscopic imaging to noninvasively evaluate in vivo delivery pathways of CSF fluorescent tracers. Tracers in CSF proved to be distributed through a brain-wide network of periarterial spaces, previously denoted as the glymphatic system. CSF tracer entry was enhanced approximately 3-fold by increasing plasma osmolality without disruption of the blood-brain barrier. Further, plasma hyperosmolality overrode the inhibition of glymphatic transport that characterizes the awake state and reversed glymphatic suppression in a mouse model of Alzheimer's disease. Plasma hyperosmolality enhanced the delivery of an amyloid-β (Aβ) antibody, obtaining a 5-fold increase in antibody binding to Aβ plaques. Thus, manipulation of glymphatic activity may represent a novel strategy for improving penetration of therapeutic antibodies to the CNS.
Keywords: Alzheimer’s disease; Immunotherapy; Neuroimaging; Neuroscience; Therapeutics.
Conflict of interest statement
Figures

References
-
- Gros-Louis F, Soucy G, Larivière R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113(5):1188–1199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

