Zika virus shedding in the stool and infection through the anorectal mucosa in mice
- PMID: 30333476
- PMCID: PMC6193040
- DOI: 10.1038/s41426-018-0170-6
Zika virus shedding in the stool and infection through the anorectal mucosa in mice
Abstract
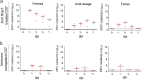
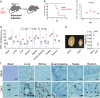
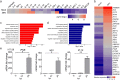
Zika virus (ZIKV) has elicited global concern due to its unique biological features, unusual transmission routes, and unexpected clinical outcomes. Although ZIKV transmission through anal intercourse has been reported in humans, it remains unclear if ZIKV is detectable in the stool, if it can infect the host through the anal canal mucosa, and what the pathogenesis of such a route of infection might be in the mouse model. Herein, we demonstrate that ZIKV RNA can be recovered from stools in multiple mouse models, as well as from the stool of a ZIKV patient. Remarkably, intra-anal (i.a.) inoculation with ZIKV leads to efficient infection in both Ifnar1-/- and immunocompetent mice, characterized by extensive viral replication in the blood and multiple organs, including the brain, small intestine, testes, and rectum, as well as robust humoral and innate immune responses. Moreover, i.a. inoculation of ZIKV in pregnant mice resulted in transplacental infection and delayed fetal development. Overall, our results identify the anorectal mucosa as a potential site of ZIKV infection in mice, reveal the associated pathogenesis of i.a. infection, and highlight the complexity of ZIKV transmission through anal intercourse.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
