Transgenic overexpression of furin increases epileptic susceptibility
- PMID: 30333479
- PMCID: PMC6193048
- DOI: 10.1038/s41419-018-1076-x
Transgenic overexpression of furin increases epileptic susceptibility
Abstract
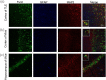
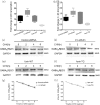
The proprotein convertase Furin plays crucial roles in the pathology of many diseases. However, the specific role of furin in epilepsy remains unclear. In our study, furin protein was increased in the temporal neocortex of epileptic patients and in the hippocampus and cortex of epileptic mice. The furin transgenic (TG) mice showed increased susceptibility to epilepsy and heightened epileptic activity compared with wild-type (WT) mice. Conversely, lentivirus-mediated knockdown of furin restrained epileptic activity. Using whole-cell patch clamp, furin knockdown and overexpression influenced neuronal inhibitory by regulating postsynaptic gamma-aminobutyric acid A receptor (GABAAR)-mediated synaptic transmission. Importantly, furin influenced the expression of GABAAR β2/3 membrane and total protein in epileptic mice by changing transcription level of GABAAR β2/3, not the protein degradation. These results reveal that furin may regulate GABAAR-mediated inhibitory synaptic transmission by altering the transcription of GABAAR β2/3 subunits in epilepsy; this finding could provide new insight into epilepsy prevention and treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

