Developmental roadmap for antimicrobial susceptibility testing systems
- PMID: 30333569
- PMCID: PMC7138758
- DOI: 10.1038/s41579-018-0098-9
Developmental roadmap for antimicrobial susceptibility testing systems
Abstract
Antimicrobial susceptibility testing (AST) technologies help to accelerate the initiation of targeted antimicrobial therapy for patients with infections and could potentially extend the lifespan of current narrow-spectrum antimicrobials. Although conceptually new and rapid AST technologies have been described, including new phenotyping methods, digital imaging and genomic approaches, there is no single major, or broadly accepted, technological breakthrough that leads the field of rapid AST platform development. This might be owing to several barriers that prevent the timely development and implementation of novel and rapid AST platforms in health-care settings. In this Consensus Statement, we explore such barriers, which include the utility of new methods, the complex process of validating new technology against reference methods beyond the proof-of-concept phase, the legal and regulatory landscapes, costs, the uptake of new tools, reagent stability, optimization of target product profiles, difficulties conducting clinical trials and issues relating to quality and quality control, and present possible solutions.
Conflict of interest statement
A.v.B., A.M. and W.M.D. are employees of bioMérieux, an international company developing, marketing and selling diagnostic tests, including antimicrobial susceptibility testing. G.L. is an employee of Curetis, a company that is active in the field of infectious disease diagnostics. bioMérieux and Curetis have had no influence on the current more personal account presented in this manuscript. J.P.H. is a partner in the European Union H202-funded project DIAGORAS (
Figures
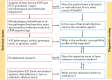

References
-
- European Centre for Disease prevention and Control (ECDC), European Medicine Agency (EMEA). The bacterial challenge: time to react (ECDC, 2009).
-
- Nault V, et al. Sustained impact of a computer-assisted antimicrobial stewardship intervention on antimicrobial use and length of stay. J. Antimicrob. Chemother. 2017;72:933–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

