LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration
- PMID: 30333625
- PMCID: PMC6296374
- DOI: 10.1038/s41586-018-0615-z
LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration
Abstract
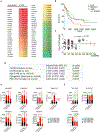
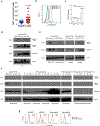
Immune checkpoint blockade therapy has been successful in treating some types of cancer but has not shown clinical benefits for treating leukaemia1. This result suggests that leukaemia uses unique mechanisms to evade this therapy. Certain immune inhibitory receptors that are expressed by normal immune cells are also present on leukaemia cells. Whether these receptors can initiate immune-related primary signalling in tumour cells remains unknown. Here we use mouse models and human cells to show that LILRB4, an immunoreceptor tyrosine-based inhibition motif-containing receptor and a marker of monocytic leukaemia, supports tumour cell infiltration into tissues and suppresses T cell activity via a signalling pathway that involves APOE, LILRB4, SHP-2, uPAR and ARG1 in acute myeloid leukaemia (AML) cells. Deletion of LILRB4 or the use of antibodies to block LILRB4 signalling impeded AML development. Thus, LILRB4 orchestrates tumour invasion pathways in monocytic leukaemia cells by creating an immunosuppressive microenvironment. LILRB4 represents a compelling target for the treatment of monocytic AML.
Figures












References
-
- Curran EK, Godfrey J & Kline J Mechanisms of Immune Tolerance in Leukemia and Lymphoma. Trends Immunol, doi:10.1016/j.it.2017.04.004 (2017). - DOI - PMC - PubMed
-
- Kang X et al. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle 15, 25–40, doi:10.1080/15384101.2015.1121324 (2016). - DOI - PMC - PubMed
-
- Hirayasu K & Arase H Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. Journal of human genetics, doi:10.1038/jhg.2015.64 (2015). - DOI - PubMed
-
- Trowsdale J, Jones DC, Barrow AD & Traherne JA Surveillance of cell and tissue perturbation by receptors in the LRC. Immunol Rev 267, 117–136, doi:10.1111/imr.12314 (2015). - DOI - PubMed
-
- Dobrowolska H et al. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry B Clin Cytom 84, 21–29, doi:10.1002/cyto.b.21050 (2013). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

