Calcium Deregulation: Novel Insights to Understand Friedreich's Ataxia Pathophysiology
- PMID: 30333728
- PMCID: PMC6176067
- DOI: 10.3389/fncel.2018.00264
Calcium Deregulation: Novel Insights to Understand Friedreich's Ataxia Pathophysiology
Abstract
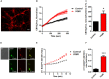
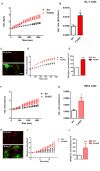
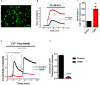
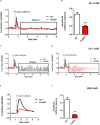
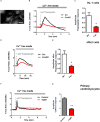
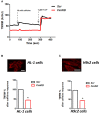
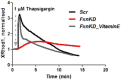
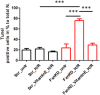
Friedreich's Ataxia (FRDA) is a neurodegenerative disorder, characterized by degeneration of dorsal root ganglia, cerebellum and cardiomyopathy. Heart failure is one of the most common causes of death for FRDA patients. Deficiency of frataxin, a small mitochondrial protein, is responsible for all clinical and morphological manifestations of FRDA. The focus of our study was to investigate the unexplored Ca2+ homeostasis in cerebellar granule neurons (CGNs) and in cardiomyocytes of FRDA cellular models to understand the pathogenesis of degeneration. Ca2+ homeostasis in neurons and cardiomyocytes is not only crucial for the cellular wellbeing but more importantly to generate action potential in both neurons and cardiomyocytes. By challenging Ca2+ homeostasis in CGNs, and in adult and neonatal cardiomyocytes of FRDA models, we have assessed the impact of frataxin decrease on both neuronal and cardiac physiopathology. Interestingly, we have found that Ca2+ homeostasis is altered both cell types. CGNs showed a Ca2+ mishandling under depolarizing conditions and this was also reflected in the endoplasmic reticulum (ER) content. In cardiomyocytes we found that the sarcoplasmic reticulum (SR) Ca2+ content was pathologically reduced, and that mitochondrial Ca2+ uptake was impaired. This phenomenon is due to the excess of oxidative stress under FRDA like conditions and the consequent aberrant modulation of key players at the SR/ER and mitochondrial level that usually restore the Ca2+ homeostasis. Our findings demonstrate that in both neurons and cardiomyocytes the decreased Ca2+ level within the stores has a comparable detrimental impact in their physiology. In cardiomyocytes, we found that ryanodine receptors (RyRs) may be leaking and expel more Ca2+ out from the SR. At the same time mitochondrial uptake was altered and we found that Vitamin E can restore this defect. Moreover, Vitamin E protects from cell death induced by hypoxia-reperfusion injury, revealing novel properties of Vitamin E as potential therapeutic tool for FRDA cardiomyopathy.
Keywords: CGNs; FRDA; calcium; cardiomyocytes; oxidative stress; ryanodine receptors and mitochondrial membrane potential; sarcoplasmic reticulum.
Figures

References
-
- Al-Mahdawi S., Pinto R. M., Varshney D., Lawrence L., Lowrie M. B., Hughes S., et al. (2006). GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88 580–590. 10.1016/j.ygeno.2006.06.015 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

