Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol
- PMID: 30333816
- PMCID: PMC6176115
- DOI: 10.3389/fmicb.2018.02363
Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol
Abstract
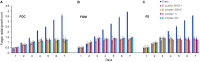
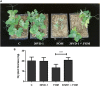
Fusarium is a complex genus of ascomycete fungi that consists of plant pathogens of agricultural relevance. Controlling Fusarium infection in crops that leads to substantial yield losses is challenging. These economic losses along with environmental and human health concerns over the usage of chemicals in attaining disease control are shifting focus toward the use of biocontrol agents for effective control of phytopathogenic Fusarium spp. In the present study, an analysis of the plant-growth promoting (PGP) and biocontrol attributes of four bacilli (Bacillus simplex 30N-5, B. simplex 11, B. simplex 237, and B. subtilis 30VD-1) has been conducted. The production of cellulase, xylanase, pectinase, and chitinase in functional assays was studied, followed by in silico gene analysis of the PGP-related and biocontrol-associated genes. Of all the bacilli included in this study, B. subtilis 30VD-1 (30VD-1) demonstrated the most effective antagonism against Fusarium spp. under in vitro conditions. Additionally, 100 μg/ml of the crude 1-butanol extract of 30VD-1's cell-free culture filtrate caused about 40% inhibition in radial growth of Fusarium spp. Pea seed bacterization with 30VD-1 led to considerable reduction in wilt severity in plants with about 35% increase in dry plant biomass over uninoculated plants growing in Fusarium-infested soil. Phase contrast microscopy demonstrated distortions and abnormal swellings in F. oxysporum hyphae on co-culturing with 30VD-1. The results suggest a multivariate mode of antagonism of 30VD-1 against phytopathogenic Fusarium spp., by producing chitinase, volatiles, and other antifungal molecules, the characterization of which is underway.
Keywords: Bacillus; Fusarium; biocontrol bacteria; hydrolytic enzymes; plant growth promoting bacteria.
Figures


References
-
- Abd El Daim I. A., Häggenblom P., Karlsson M., Strenstrom E., Timmusk S. (2015). Paenibacillus polymyxa A26 Sfp-type PPTase inactivation limits bacterial antagonism against Fusarium graminearum but not of F. culmorum in kernel assay. Front. Plant Sci. 6:368. 10.3389/fpls.2015.00368 - DOI - PMC - PubMed
-
- Arrebola E., Sivakum D., Korsten L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biocontrol 53 122–123.
-
- Aydi Ben Abdallah R., Jabnoun-Khlareddine H., Nefzi A., Mokni-Tlili S., Daami-Remadi M. (2016). Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Solanum elaeagnifolium stems. J. Phytopathol. 164 811–824. 10.1111/jph.12501 - DOI
-
- Bashan Y., Holguin G. (1998). Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 30 1225–1228. 10.1016/s0038-0717(97)00187-9 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

