iNKT Cells Suppress Pathogenic NK1.1+CD8+ T Cells in DSS-Induced Colitis
- PMID: 30333822
- PMCID: PMC6176072
- DOI: 10.3389/fimmu.2018.02168
iNKT Cells Suppress Pathogenic NK1.1+CD8+ T Cells in DSS-Induced Colitis
Abstract
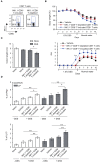
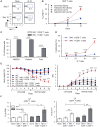
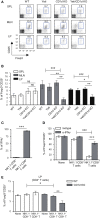
T cells producing IFNγ play a pathogenic role in the development of inflammatory bowel disease (IBD). To investigate the functions of CD1d-dependent invariant natural killer T (iNKT) cells in experimental colitis induced in Yeti mice with dysregulated expression of IFNγ, we generated iNKT cell-deficient Yeti/CD1d KO mice and compared colitis among WT, CD1d KO, Yeti, and Yeti/CD1d KO mice following DSS treatment. We found that deficiency of iNKT cells exacerbated colitis and disease pathogenesis was mainly mediated by NK1.1+CD8+ T cells. Furthermore, the protective effects of iNKT cells correlated with up-regulation of regulatory T cells. Taken together, our results have demonstrated that CD1d-dependent iNKT cells and CD1d-independent NK1.1+CD8+ T cells reciprocally regulate the development of intestinal inflammatory responses mediated by IFNγ-dysregulation. These findings also identify NK1.1+CD8+ T cells as novel target cells for the development of therapeutics for human IBD.
Keywords: CD1d-dependent NKT cells; DSS-induced colitis; IFNγ; NK1.1+CD8+ T cells; Treg cells.
Figures



Similar articles
-
Opposing Roles of DCs and iNKT Cells in the Induction of Foxp3 Expression by MLN CD25+CD4+ T Cells during IFNγ-Driven Colitis.Int J Mol Sci. 2022 Dec 5;23(23):15316. doi: 10.3390/ijms232315316. Int J Mol Sci. 2022. PMID: 36499642 Free PMC article.
-
CD1d-Dependent iNKT Cells Control DSS-Induced Colitis in a Mouse Model of IFNγ-Mediated Hyperinflammation by Increasing IL22-Secreting ILC3 Cells.Int J Mol Sci. 2021 Jan 27;22(3):1250. doi: 10.3390/ijms22031250. Int J Mol Sci. 2021. PMID: 33513946 Free PMC article.
-
NKT Cell-Deficient Mice Harbor an Altered Microbiota That Fuels Intestinal Inflammation during Chemically Induced Colitis.J Immunol. 2016 Dec 1;197(11):4464-4472. doi: 10.4049/jimmunol.1601410. Epub 2016 Oct 31. J Immunol. 2016. PMID: 27799307
-
Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control.Front Immunol. 2018 Jun 6;9:1266. doi: 10.3389/fimmu.2018.01266. eCollection 2018. Front Immunol. 2018. PMID: 29928278 Free PMC article. Review.
-
The Role of Invariant NKT in Autoimmune Liver Disease: Can Vitamin D Act as an Immunomodulator?Can J Gastroenterol Hepatol. 2018 Jun 26;2018:8197937. doi: 10.1155/2018/8197937. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 30046564 Free PMC article. Review.
Cited by
-
Repeated α-GalCer Administration Induces a Type 2 Cytokine-Biased iNKT Cell Response and Exacerbates Atopic Skin Inflammation in Vα14Tg NC/Nga Mice.Biomedicines. 2021 Nov 4;9(11):1619. doi: 10.3390/biomedicines9111619. Biomedicines. 2021. PMID: 34829848 Free PMC article.
-
TLR4-dependent effects of ISAg treatment on conventional T cell polarization in vivo.Anim Cells Syst (Seoul). 2019 Apr 25;23(3):184-191. doi: 10.1080/19768354.2019.1610059. eCollection 2019 Jun. Anim Cells Syst (Seoul). 2019. PMID: 31231582 Free PMC article.
-
Opposing Roles of DCs and iNKT Cells in the Induction of Foxp3 Expression by MLN CD25+CD4+ T Cells during IFNγ-Driven Colitis.Int J Mol Sci. 2022 Dec 5;23(23):15316. doi: 10.3390/ijms232315316. Int J Mol Sci. 2022. PMID: 36499642 Free PMC article.
-
CD1d-independent NK1.1+ Treg cells are IL2-inducible Foxp3+ T cells co-expressing immunosuppressive and cytotoxic molecules.Front Immunol. 2022 Sep 13;13:951592. doi: 10.3389/fimmu.2022.951592. eCollection 2022. Front Immunol. 2022. PMID: 36177042 Free PMC article.
-
CD8+ T-cell immunity orchestrated by iNKT cells.Front Immunol. 2023 Jan 18;13:1109347. doi: 10.3389/fimmu.2022.1109347. eCollection 2022. Front Immunol. 2023. PMID: 36741397 Free PMC article. Review.
References
-
- Park HJ, Lee SW, Kwon DJ, Heo SI, Park SH, Kim SY, et al. . Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses. BMC Complement Altern Med. (2017) 17:448. 10.1186/s12906-017-1952-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

