Metabolite-Sensing G Protein Coupled Receptor TGR5 Protects Host From Viral Infection Through Amplifying Type I Interferon Responses
- PMID: 30333836
- PMCID: PMC6176213
- DOI: 10.3389/fimmu.2018.02289
Metabolite-Sensing G Protein Coupled Receptor TGR5 Protects Host From Viral Infection Through Amplifying Type I Interferon Responses
Abstract
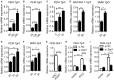
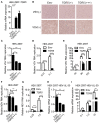
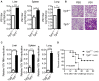
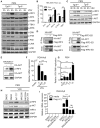
The metabolite-sensing G protein-coupled receptors (GPCRs) bind to various metabolites and transmit signals that are important for proper immune and metabolic functions. However, the roles of metabolite-sensing GPCRs in viral infection are not well characterized. Here, we identified metabolite-sensing GPCR TGR5 as an interferon (IFN)-stimulated gene (ISG) which had increased expression following viral infection or IFN-β stimulation in a STAT1-dependent manner. Most importantly, overexpression of TGR5 or treatment with the modified bile acid INT-777 broadly protected host cells from vesicular stomatitis virus (VSV), newcastle disease virus (NDV) and herpes simplex virus type 1 (HSV-1) infection. Furthermore, VSV and HSV-1 replication was increased significantly in Tgr5-deficient macrophages and the VSV distribution in liver, spleen and lungs was increased in Tgr5-deficient mice during VSV infection. Accordingly, Tgr5-deficient mice were much more susceptible to VSV infection than wild-type mice. Mechanistically, TGR5 facilitates type I interferon (IFN-I) production through the AKT/IRF3-signaling pathway, which is crucial in promoting antiviral innate immunity. Taken together, our data reveal a positive feedback loop regulating IRF3 signaling and suggest a potential therapeutic role for metabolite-sensing GPCRs in controlling viral diseases.
Keywords: IFN-I; ISG; TGR5; metabolite-sensing GPCRs; viral infection.
Figures


Similar articles
-
GNAQ Negatively Regulates Antiviral Innate Immune Responses in a Calcineurin-Dependent Manner.J Immunol. 2019 Sep 1;203(5):1288-1297. doi: 10.4049/jimmunol.1900427. Epub 2019 Jul 19. J Immunol. 2019. PMID: 31324725
-
Elimination of GPR146-mediated antiviral function through IRF3/HES1-signalling pathway.Immunology. 2017 Sep;152(1):102-114. doi: 10.1111/imm.12752. Epub 2017 Jun 1. Immunology. 2017. PMID: 28464285 Free PMC article.
-
Extracellular UDP and P2Y6 function as a danger signal to protect mice from vesicular stomatitis virus infection through an increase in IFN-β production.J Immunol. 2014 Nov 1;193(9):4515-26. doi: 10.4049/jimmunol.1301930. Epub 2014 Sep 26. J Immunol. 2014. PMID: 25261483
-
Herpes Simplex Virus Type 1 and Host Antiviral Immune Responses: An Update.Viral Immunol. 2019 Dec;32(10):424-429. doi: 10.1089/vim.2019.0097. Epub 2019 Oct 10. Viral Immunol. 2019. PMID: 31599707 Review.
-
Metabolite-Sensing G Protein-Coupled Receptors-Facilitators of Diet-Related Immune Regulation.Annu Rev Immunol. 2017 Apr 26;35:371-402. doi: 10.1146/annurev-immunol-051116-052235. Annu Rev Immunol. 2017. PMID: 28446062 Review.
Cited by
-
Virus-Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays.Viruses. 2023 Dec 12;15(12):2412. doi: 10.3390/v15122412. Viruses. 2023. PMID: 38140653 Free PMC article.
-
G protein-coupled bile acid receptor 1 reduced hepatic immune response and inhibited NFκB, PI3K/AKT, and PKC/P38 MAPK signaling pathway in hybrid grouper.J Anim Sci. 2023 Jan 3;101:skad307. doi: 10.1093/jas/skad307. J Anim Sci. 2023. PMID: 37715969 Free PMC article.
-
TGR5 deficiency activates antitumor immunity in non-small cell lung cancer via restraining M2 macrophage polarization.Acta Pharm Sin B. 2022 Feb;12(2):787-800. doi: 10.1016/j.apsb.2021.07.011. Epub 2021 Jul 21. Acta Pharm Sin B. 2022. PMID: 35256947 Free PMC article.
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
-
The gut microbiota-bile acid axis: a crucial regulator of immune function and metabolic health.World J Microbiol Biotechnol. 2025 Jun 25;41(7):215. doi: 10.1007/s11274-025-04395-7. World J Microbiol Biotechnol. 2025. PMID: 40555888 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

