Terminal Alkenes from Acrylic Acid Derivatives via Non-Oxidative Enzymatic Decarboxylation by Ferulic Acid Decarboxylases
- PMID: 30333895
- PMCID: PMC6175315
- DOI: 10.1002/cctc.201800643
Terminal Alkenes from Acrylic Acid Derivatives via Non-Oxidative Enzymatic Decarboxylation by Ferulic Acid Decarboxylases
Abstract
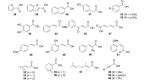
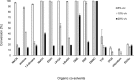
Fungal ferulic acid decarboxylases (FDCs) belong to the UbiD-family of enzymes and catalyse the reversible (de)carboxylation of cinnamic acid derivatives through the use of a prenylated flavin cofactor. The latter is synthesised by the flavin prenyltransferase UbiX. Herein, we demonstrate the applicability of FDC/UbiX expressing cells for both isolated enzyme and whole-cell biocatalysis. FDCs exhibit high activity with total turnover numbers (TTN) of up to 55000 and turnover frequency (TOF) of up to 370 min-1. Co-solvent compatibility studies revealed FDC's tolerance to some organic solvents up 20 % v/v. Using the in-vitro (de)carboxylase activity of holo-FDC as well as whole-cell biocatalysts, we performed a substrate profiling study of three FDCs, providing insights into structural determinants of activity. FDCs display broad substrate tolerance towards a wide range of acrylic acid derivatives bearing (hetero)cyclic or olefinic substituents at C3 affording conversions of up to >99 %. The synthetic utility of FDCs was demonstrated by a preparative-scale decarboxylation.
Keywords: Biocatalysis; Decarboxylation; Ferulic acid decarboxylase; Prenylated flavin; Terminal alkenes.
Figures
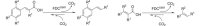



Similar articles
-
Surveying the scope of aromatic decarboxylations catalyzed by prenylated-flavin dependent enzymes.Faraday Discuss. 2024 Sep 11;252(0):208-222. doi: 10.1039/d4fd00006d. Faraday Discuss. 2024. PMID: 38837123
-
Structural insights into UbiD reversible decarboxylation.Curr Opin Struct Biol. 2022 Aug;75:102432. doi: 10.1016/j.sbi.2022.102432. Epub 2022 Jul 14. Curr Opin Struct Biol. 2022. PMID: 35843126 Review.
-
Isofunctional enzymes PAD1 and UbiX catalyze formation of a novel cofactor required by ferulic acid decarboxylase and 4-hydroxy-3-polyprenylbenzoic acid decarboxylase.ACS Chem Biol. 2015 Apr 17;10(4):1137-44. doi: 10.1021/cb5008103. Epub 2015 Feb 3. ACS Chem Biol. 2015. PMID: 25647642 Free PMC article.
-
Ferulic Acid Decarboxylase Controls Oxidative Maturation of the Prenylated Flavin Mononucleotide Cofactor.ACS Chem Biol. 2020 Sep 18;15(9):2466-2475. doi: 10.1021/acschembio.0c00456. Epub 2020 Sep 8. ACS Chem Biol. 2020. PMID: 32840348
-
Prenylated flavins: structures and mechanisms.FEBS J. 2023 May;290(9):2232-2245. doi: 10.1111/febs.16371. Epub 2022 Feb 4. FEBS J. 2023. PMID: 35073609 Review.
Cited by
-
Rapid prototyping of microbial production strains for the biomanufacture of potential materials monomers.Metab Eng. 2020 Jul;60:168-182. doi: 10.1016/j.ymben.2020.04.008. Epub 2020 Apr 23. Metab Eng. 2020. PMID: 32335188 Free PMC article.
-
Non-Oxidative Enzymatic (De)Carboxylation of (Hetero)Aromatics and Acrylic Acid Derivatives.Adv Synth Catal. 2019 Jun 6;361(11):2402-2420. doi: 10.1002/adsc.201900275. Epub 2019 May 17. Adv Synth Catal. 2019. PMID: 31379472 Free PMC article. Review.
-
Cinnamic Acid and Sorbic acid Conversion Are Mediated by the Same Transcriptional Regulator in Aspergillus niger.Front Bioeng Biotechnol. 2019 Sep 27;7:249. doi: 10.3389/fbioe.2019.00249. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31612133 Free PMC article.
-
Direct 1,3-butadiene biosynthesis in Escherichia coli via a tailored ferulic acid decarboxylase mutant.Nat Commun. 2021 Apr 13;12(1):2195. doi: 10.1038/s41467-021-22504-6. Nat Commun. 2021. PMID: 33850144 Free PMC article.
-
Enzymatic Conversion of CO2: From Natural to Artificial Utilization.Chem Rev. 2023 May 10;123(9):5702-5754. doi: 10.1021/acs.chemrev.2c00581. Epub 2023 Jan 24. Chem Rev. 2023. PMID: 36692850 Free PMC article. Review.
References
-
- Tinikul R., Chenprakhon P., Maenpuen S., Chaiyen P., Biotechnol. J. 2018, DOI 10.1002/biot.201700632. - PubMed
-
- Kourist R., Guterl J.-K., Miyamoto K., Sieber V., ChemCatChem 2014, 6, 689–701.
-
- Fesko K., Gruber‐Khadjawi M., ChemCatChem 2013, 5, 1248–1272.
-
- Glueck S. M., Gümüs S., Fabian W. M. F., Faber K., Chem. Soc. Rev. 2010, 39, 313–328. - PubMed
-
- Ren T., Patel M., Blok K., Energy 2006, 31, 425–451.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
