Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3
- PMID: 30333912
- PMCID: PMC6173462
- DOI: 10.18632/oncotarget.26107
Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3
Abstract
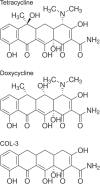
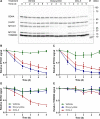
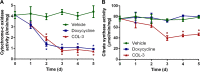
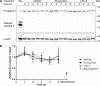
Tetracyclines have anticancer properties in addition to their well-known antibacterial properties. It has been proposed that tetracyclines slow metastasis and angiogenesis through inhibition of matrix metalloproteinases. However, we believe that the anticancer effect of tetracyclines is due to their inhibition of mitochondrial protein synthesis, resulting in a decrease of the mitochondrial energy generating capacity. Several groups have developed analogs that are void of antibacterial action. An example is COL-3, which is currently tested for its anticancer effects in clinical trials. We have undertaken a comparative study of the tetracycline analogs COL-3 and doxycycline, which has an antibacterial function, to further investigate the role of the mitochondrial energy generating capacity in the anticancer mechanism and, thereby, evaluate the usefulness of mitochondria as an oncotarget. Our experiments with cultures of the human A549, COLO357 and HT29 cancer cells and fibroblasts indicated that COL-3 is significantly more cytotoxic than doxycycline. Mitochondrial translation assays demonstrated that COL-3 has retained its inhibitory effect on mitochondrial protein synthesis. Both drugs caused a severe decrease in the levels of mitochondrially encoded cytochrome-c oxidase subunits and cytochrome-c oxidase activity. In addition, COL-3 produced a marked drop in the level of nuclear-encoded succinate dehydrogenase subunit A and citrate synthase activity, indicating that COL-3 has multiple inhibitory effects. Contrary to COL-3, the anticancer action of doxycycline appears to be based specifically on inhibition of mitochondrial protein synthesis, which is thought to affect rapidly proliferating cancer cells more than healthy tissue. Doxycycline is likely to cause less side effects that COL-3.
Keywords: COL-3; cancer; doxycycline; mitochondria; tetracycline.
Conflict of interest statement
CONFLICTS OF INTEREST Dr Jan-Willem Taanman. serves on the Scientific Advisory Board of Novintum Bioscience Ltd. The other authors have no conflict of interests to declare. No commercial entities had any role in the generation of the data contained in this paper or the preparation of the manuscript.
Figures



Similar articles
-
Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells.Int J Cancer. 2006 Mar 1;118(5):1309-15. doi: 10.1002/ijc.21447. Int J Cancer. 2006. PMID: 16152604
-
Cytotoxic effects of tetracycline analogues (doxycycline, minocycline and COL-3) in acute myeloid leukemia HL-60 cells.PLoS One. 2014 Dec 15;9(12):e114457. doi: 10.1371/journal.pone.0114457. eCollection 2014. PLoS One. 2014. PMID: 25502932 Free PMC article.
-
Doxycycline and other tetracyclines in the treatment of bone metastasis.Anticancer Drugs. 2003 Nov;14(10):773-8. doi: 10.1097/00001813-200311000-00001. Anticancer Drugs. 2003. PMID: 14597870 Review.
-
Arrest of the proliferation of renal and prostate carcinomas of human origin by inhibition of mitochondrial protein synthesis.Cancer Res. 1986 Jul;46(7):3283-9. Cancer Res. 1986. PMID: 3011245
-
[Clinical use of tetracyclines in the treatment of periodontal diseases].Med Pregl. 2000 May-Jun;53(5-6):266-71. Med Pregl. 2000. PMID: 11089368 Review. Croatian.
Cited by
-
Altered Mitochondria Functionality Defines a Metastatic Cell State in Lung Cancer and Creates an Exploitable Vulnerability.Cancer Res. 2021 Feb 1;81(3):567-579. doi: 10.1158/0008-5472.CAN-20-1865. Epub 2020 Nov 25. Cancer Res. 2021. PMID: 33239425 Free PMC article.
-
Computational drug discovery for castration-resistant prostate cancers through in vitro drug response modeling.Proc Natl Acad Sci U S A. 2023 Apr 25;120(17):e2218522120. doi: 10.1073/pnas.2218522120. Epub 2023 Apr 17. Proc Natl Acad Sci U S A. 2023. PMID: 37068243 Free PMC article.
-
Muscle biochemistry and residue accretion in male Oreochromis niloticus fries administered therapeutic, subtherapeutic and overdoses of dietary oxytetracycline.Fish Physiol Biochem. 2025 Jan 18;51(1):35. doi: 10.1007/s10695-024-01447-x. Fish Physiol Biochem. 2025. PMID: 39827248
-
Deregulation of mitochondrial gene expression in cancer: mechanisms and therapeutic opportunities.Br J Cancer. 2024 Nov;131(9):1415-1424. doi: 10.1038/s41416-024-02817-1. Epub 2024 Aug 14. Br J Cancer. 2024. PMID: 39143326 Free PMC article. Review.
-
Mitochondrial ribosomal proteins in metastasis and their potential use as prognostic and therapeutic targets.Cancer Metastasis Rev. 2024 Dec;43(4):1119-1135. doi: 10.1007/s10555-024-10216-4. Epub 2024 Oct 1. Cancer Metastasis Rev. 2024. PMID: 39354291 Free PMC article. Review.
References
-
- Hermann T. Chemical and functional diversity of small molecule ligands for RNA. Biopolymers. 2003;70:4–18. - PubMed
-
- Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58:256–265. - PubMed
-
- Kroon AM, van den Bogert C. The mitochondrial genome as a target for chemotherapy of cancer. In: Quagliarriello E, Slater EC, Palmieri F, Saccone C, Kroon AM, editors. Achievements and perspectives of mitochondrial research 2, 21–33; 1985, Elsevier, Amsterdam.
-
- van den Bogert C, van Kernebeek G, de Leij L, Kroon AM. Inhibition of mitochondrial protein synthesis leads to proliferation arrest in the G1-phase of the cell cycle. Cancer Lett. 1986;32:41–51. - PubMed
-
- Onoda T, Ono T, Dhar DK, Yamanoi A, Nagasue N. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int J Cancer. 2006;118:1309–1315. - PubMed
LinkOut - more resources
Full Text Sources

