Electrolyte and Acid-Base Disorders in the Renal Transplant Recipient
- PMID: 30333977
- PMCID: PMC6176109
- DOI: 10.3389/fmed.2018.00261
Electrolyte and Acid-Base Disorders in the Renal Transplant Recipient
Abstract
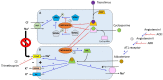
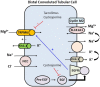
Kidney transplantation is the current treatment of choice for patients with end-stage renal disease. Innovations in transplantation and immunosuppression regimens have greatly improved the renal allograft survival. Based on recently published data from the Scientific Registry of Transplant recipients, prevalence of kidney transplants is steadily rising in the United States. Over 210,000 kidney transplant recipients were alive with a functioning graft in mid-2016, which is nearly twice as many as in 2005. While successful renal transplantation corrects most of the electrolyte and mineral abnormalities seen in advanced renal failure, the abnormalities seen in the post-transplant period are surprisingly different from those seen in chronic kidney disease. Multiple factors contribute to the high prevalence of these abnormalities that include level of allograft function, use of immunosuppressive medications and metabolic changes in the post-transplant period. Electrolyte disturbances are common in patients after renal transplantation, and several studies have tried to determine the clinical significance of these disturbances. In this manuscript we review the key aspects of the most commonly found post-transplant electrolyte abnormalities. We focus on their epidemiology, pathophysiology, clinical manifestations, and available treatment approaches.
Keywords: electrolytes; hypercalcemia; hyperkalemia; hypomagnesemia; hypophosphatemia; kidney transplant; metabolic acidosis; renal transplant.
Figures
Similar articles
-
Electrolyte and Acid-Base Abnormalities After Kidney Transplantation.Adv Kidney Dis Health. 2024 Sep;31(5):450-457. doi: 10.1053/j.akdh.2024.03.007. Adv Kidney Dis Health. 2024. PMID: 39232615 Review.
-
Electrolytes disturbances after kidney transplantation.Acta Clin Belg. 2019 Feb;74(1):48-52. doi: 10.1080/17843286.2018.1549193. Epub 2018 Nov 27. Acta Clin Belg. 2019. PMID: 30482110
-
Electrolyte disorders in stable renal allograft recipients.Swiss Med Wkly. 2020 Nov 12;150:w20366. doi: 10.4414/smw.2020.20366. eCollection 2020 Oct 19. Swiss Med Wkly. 2020. PMID: 33181857
-
Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction.Exp Clin Transplant. 2010 Dec;8(4):277-82. Exp Clin Transplant. 2010. PMID: 21143092
-
Pathogenesis and treatment of electrolyte problems post transplant.Curr Opin Pediatr. 2019 Apr;31(2):213-218. doi: 10.1097/MOP.0000000000000715. Curr Opin Pediatr. 2019. PMID: 30585865 Review.
Cited by
-
Clinical factors associated with severe hypophosphataemia after kidney transplant.BMC Nephrol. 2021 Dec 9;22(1):407. doi: 10.1186/s12882-021-02624-3. BMC Nephrol. 2021. PMID: 34886802 Free PMC article.
-
Pre-Transplant Plasma Potassium as a Potential Risk Factor for the Need of Early Hyperkalaemia Treatment after Kidney Transplantation: A Cohort Study.Nephron. 2021;145(1):63-70. doi: 10.1159/000511404. Epub 2020 Nov 19. Nephron. 2021. PMID: 33212442 Free PMC article.
-
Hyperkalaemia in Cardiological Patients: New Solutions for an Old Problem.Cardiovasc Drugs Ther. 2025 Aug;39(4):857-874. doi: 10.1007/s10557-024-07551-7. Epub 2024 Jan 30. Cardiovasc Drugs Ther. 2025. PMID: 38289453 Review.
-
Clot stones in transplant kidneys.BMJ Case Rep. 2024 Jan 5;17(1):e256843. doi: 10.1136/bcr-2023-256843. BMJ Case Rep. 2024. PMID: 38182171
-
Choroidal Vascularity Index and Choroidal Thickness Changes Following Renal Transplantation.Turk J Ophthalmol. 2023 Apr 20;53(2):97-104. doi: 10.4274/tjo.galenos.2022.02489. Turk J Ophthalmol. 2023. PMID: 37089020 Free PMC article.
References
-
- Jones JW, Gruessner RW, Gores PF, Matas AJ. Hypoaldosteronemic hyporeninemic hyperkalemia after renal transplantation. Transplantation (1993) 56:1013–5. - PubMed
-
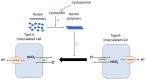
- Higgins R, Ramaiyan K, Dasgupta T, Kanji H, Fletcher S, Lam F, et al. . Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol Dial Transplant. (2004) 19:444–50. 10.1093/ndt/gfg515 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

