Transforming the Perioperative Treatment Paradigm in Non-Metastatic RCC-A Possible Path Forward
- PMID: 30334002
- PMCID: PMC6179104
- DOI: 10.3233/KCA-170010
Transforming the Perioperative Treatment Paradigm in Non-Metastatic RCC-A Possible Path Forward
Abstract
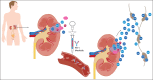
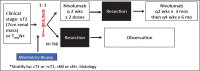
In 2017, there is no adjuvant systemic therapy proven to increase overall survival in non-metastatic renal cell carcinoma (RCC). The anti-PD-1 antibody nivolumab improves overall survival in metastatic treatment refractory RCC and is generally tolerable. Mouse solid tumor models have revealed a benefit with a short course of neoadjuvant PD-1 blockade compared to adjuvant therapy. Two ongoing phase 2 studies of perioperative nivolumab in RCC patients have shown preliminary feasibility and safety with no surgical delays or complications. The recently opened PROSPER RCC trial (A Phase 3 RandOmized Study Comparing PERioperative Nivolumab vs. Observation in Patients with Localized Renal Cell Carcinoma Undergoing Nephrectomy; EA8143) will examine if the addition of perioperative nivolumab to radical or partial nephrectomy can improve clinical outcomes in patients with high risk localized and locally advanced RCC. With the goal of increasing cure and recurrence-free survival (RFS) rates in non-metastatic RCC, we are executing a three-pronged, multidisciplinary approach of presurgical priming with nivolumab followed by resection and adjuvant PD-1 blockade. We plan to enroll 766 patients with clinical stage ≥T2 or node positive M0 RCC of any histology in this global, randomized, unblinded, phase 3 National Clinical Trials Network study. The investigational arm will receive two doses of nivolumab 240 mg IV prior to surgery followed by adjuvant nivolumab for 9 months. The control arm will undergo the current standard of care: surgical resection followed by observation. Patients are stratified by clinical T stage, node positivity, and histology. The trial is powered to detect a 14.4% absolute benefit in the primary endpoint of RFS from the ASSURE historical control of 55.8% to 70.2% at 5 years (HR = 0.70). The study is also powered to detect a significant overall survival benefit (HR 0.67). Key safety, feasibility, and quality of life endpoints are incorporated. PROSPER RCC exemplifies team science with a host of planned correlative work to investigate the impact of the baseline immune milieu and changes after neoadjuvant priming on clinical outcomes.
Keywords: EA8143; PD-1 blockade; PROSPER RCC; Renal cell carcinoma; adjuvant; neoadjuvant; nephrectomy; nivolumab; priming; recurrence-free survival.
Figures
Similar articles
-
The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER?Future Oncol. 2019 May;15(15):1683-1695. doi: 10.2217/fon-2018-0951. Epub 2019 Apr 10. Future Oncol. 2019. PMID: 30968729 Free PMC article. Clinical Trial.
-
Adjuvant Therapy Options in Renal Cell Carcinoma: Where Do We Stand?Curr Treat Options Oncol. 2019 May 3;20(5):44. doi: 10.1007/s11864-019-0639-0. Curr Treat Options Oncol. 2019. PMID: 31054006 Review.
-
Phase II Study of Neoadjuvant Nivolumab in Patients with Locally Advanced Clear Cell Renal Cell Carcinoma Undergoing Nephrectomy.Eur Urol. 2022 Jun;81(6):570-573. doi: 10.1016/j.eururo.2022.01.043. Epub 2022 Feb 17. Eur Urol. 2022. PMID: 35183395 Free PMC article. Clinical Trial.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
Neoadjuvant therapy with immunoagent (nivolumab) or placebo plus chemotherapy followed by surgery and adjuvant treatment in subjects with resectable esophageal squamous cell carcinoma: study protocol of a randomized, multicenter, double blind, phase II trial (NATION-2203 trial).J Thorac Dis. 2023 Feb 28;15(2):718-730. doi: 10.21037/jtd-22-1789. Epub 2023 Feb 8. J Thorac Dis. 2023. PMID: 36910109 Free PMC article.
Cited by
-
Efficacy of presurgical therapy with tislelizumab and axitinib to downsize local lesions in locally advanced and metastatic renal cell carcinoma: a single-institution experience with long-term follow-up.Transl Androl Urol. 2025 Mar 30;14(3):651-660. doi: 10.21037/tau-24-585. Epub 2025 Mar 26. Transl Androl Urol. 2025. PMID: 40226082 Free PMC article.
-
The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER?Future Oncol. 2019 May;15(15):1683-1695. doi: 10.2217/fon-2018-0951. Epub 2019 Apr 10. Future Oncol. 2019. PMID: 30968729 Free PMC article. Clinical Trial.
-
"PROBE"ing the Role of Cytoreductive Nephrectomy in Advanced Renal Cancer.Kidney Cancer J. 2022 Mar 15;6(1):3-9. doi: 10.3233/kca-210010. Kidney Cancer J. 2022. PMID: 35310961 Free PMC article.
-
A Real-World, Population-Based Retrospective Analysis of Therapeutic Survival for Recurrent Localized Renal Cell Carcinoma After Nephrectomy.Front Oncol. 2021 Sep 8;11:693831. doi: 10.3389/fonc.2021.693831. eCollection 2021. Front Oncol. 2021. PMID: 34568023 Free PMC article.
-
The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma.Cancers (Basel). 2023 Dec 17;15(24):5873. doi: 10.3390/cancers15245873. Cancers (Basel). 2023. PMID: 38136417 Free PMC article. Review.
References
-
- Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 2001;19:1649–57. - PubMed
-
- Harshman LXW, Moreira RB, Bosse D, Ares GR, Sweeney C, Choueiri TK. Evaluation of disease-free survival as an intermediate metric for overall survival in localized renal cell carcinoma: A trial-level meta-analysis. J Clin Oncol 2017;35(suppl; abstr 4585). - PubMed
-
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016. - PubMed
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354–66. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
