Parallel (Randomized) Phase II Evaluation of Tivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOG S1107
- PMID: 30334014
- PMCID: PMC6179121
- DOI: 10.3233/KCA-170018
Parallel (Randomized) Phase II Evaluation of Tivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOG S1107
Abstract
Background: Papillary renal cell carcinoma (pRCC) is associated with EGFR expression and activation of MET signaling pathway. A randomized multicenter parallel two-stage phase II trial of MET inhibitor tivantinib alone or in combination with EGFR inhibitor erlotinib was conducted in patients with pRCC.
Methods: Patients with advanced pRCC and 0-1 prior systemic therapy were randomly assigned to tivantinib 360 mg BID (Arm 1) or tivantinib 360 mg BID plus erlotinib 150 mg daily (Arm 2). Target max accrual was 70 patients (35 per arm) with interim analysis planned after enrollment of 20 patients per arm.
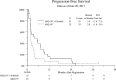
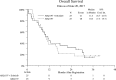
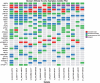
Results: Six % of patients had type 1 pRCC, 42% had type 2, and 52% had no subtype assigned. The study was closed after the first stage when both arms yielded RR of 0%. Median progression free survival (PFS) was 2.0 and 3.9 months, and OS was 10.3 and 11.3 months in Arms 1 and 2 respectively. Treatment was well tolerated. Exome of tumor tissue from 16 patients were successfully sequenced using Agilent SureSelect probes. Only 1 of 16 samples harbored MET mutation. Other mutations associated primarily with type 2 pRCC were noted and included CDKN2A, PBRM1, SETD2, KDM6A, FAT1 and NF2.
Conclusions: Tivantinib - either alone or in combination with erlotinib has no clinical activity in patients with advanced pRCC. The S1107 cohort had a low proportion of patients with MET alterations. MET remains a reasonable therapeutic target in pRCC, but selection of patient subsets exhibiting MET activation may be required to better benefit from therapy with MET inhibitors.
Keywords: EGFR inhibitor; MET inhibitor; Papillary kidney cancer; erlotinib; tivantinib.
Figures
References
-
- Rini BI, Cambell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119–32. - PubMed
-
- Pai A, Brunson A, Brown M, et al. Evolving epidemiologic trends in nonclear cell renal cell cancer: An analysis of the California Cancer Registry. Urology 2013;82(4):840–5. - PubMed
-
- Twardowski PW, Mack PC, Lara PN Jr.. Papillary renal cell carcinoma: Current progress and future directions. Clin Genitourin Cancer 2014;12(2):74–9. - PubMed
-
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915–25. - PubMed
-
- Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013;39:793–801. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous