Secukinumab is Superior to Ustekinumab in Clearing Skin in Patients with Moderate to Severe Plaque Psoriasis (16-Week CLARITY Results)
- PMID: 30334147
- PMCID: PMC6261116
- DOI: 10.1007/s13555-018-0265-y
Secukinumab is Superior to Ustekinumab in Clearing Skin in Patients with Moderate to Severe Plaque Psoriasis (16-Week CLARITY Results)
Abstract
Introduction: Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, has demonstrated superior efficacy to ustekinumab in the phase 3b CLEAR study of moderate to severe plaque psoriasis. Here, we report 16-week results from CLARITY, a second head-to-head trial comparing secukinumab with ustekinumab.
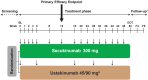
Methods: In the phase 3b CLARITY study, patients were randomized 1:1 to receive subcutaneous secukinumab 300 mg or ustekinumab per label. The co-primary objectives were to demonstrate the superiority of secukinumab over ustekinumab at Week 12 in relation to the proportion of patients with (1) 90% or more improvement from baseline Psoriasis Area and Severity Index (PASI 90) and (2) a score of 0/1 (clear/almost clear) on the modified Investigator's Global Assessment (IGA mod 2011 0/1). Key secondary objectives were also assessed, as was Dermatology Life Quality Index (DLQI) 0/1 (no impact of skin disease on patients' quality of life) response. Missing values were handled by multiple imputation except for DLQI 0/1, where last observation carried forward techniques were utilized.
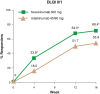
Results: Both co-primary objectives were met: secukinumab was superior to ustekinumab for the proportion of patients achieving a PASI 90 (66.5% vs. 47.9%) and IGA mod 2011 0/1 response (72.3% vs. 55.4%) at Week 12 (p < 0.0001). PASI 90 responses were greater with secukinumab compared to ustekinumab from as early as Week 4 (16.7% vs. 4.0%) and out to Week 16 (76.6% vs. 54.2%). Similarly, IGA mod 2011 0/1 findings were greater with secukinumab at Week 4 (26.9% vs. 7.8%) and at Week 16 (78.6% vs. 59.1%). DLQI 0/1 response rates were also greater with secukinumab compared to ustekinumab at Week 4 (33.9% vs. 18.0%), Week 12 (64.0% vs. 51.7%), and Week 16 (68.4% vs. 55.9%).
Conclusion: The results of this study confirm the superior efficacy of secukinumab over ustekinumab in treating patients with moderate to severe psoriasis.
Trial registration: Clinicaltrials.gov Identifier, NCT02826603.
Funding: Novartis Pharma AG, Basel, Switzerland.
Keywords: Moderate to severe psoriasis; Secukinumab; Ustekinumab.
Figures

References
-
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. - DOI - PubMed
-
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

